1. Analysis of baseline records
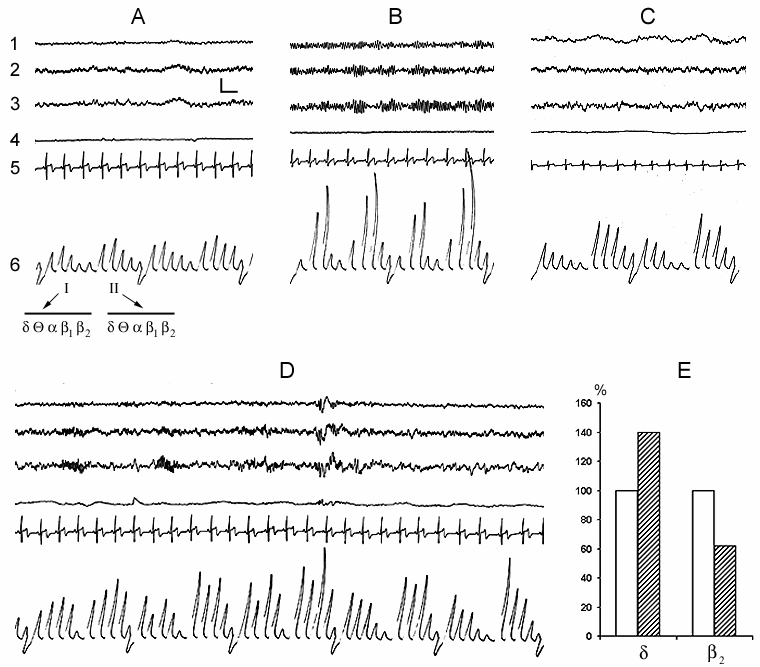
In spite of the fact that the subject made himself/herself comfortable in bed with the intention to fall asleep, a definite time (a few minutes) after having taken convenient posture for sleep he/she spends with eyes open. At this time the EEG shows the phenomenon of obvious desynchronization, i.e. all the basic slow rhythms (delta, theta, alpha) are suppressed (Fig. 1A). It is natural that at this time, in spite of general relaxation of the body, tonic activity of skeletal muscles is maintained on a definite level. Cardiac rhythm also keeps on a certain level, although it is significantly lower than at more active wakefulness. There is no eye movement as the subject in a semi-dark room does not make observation on any objects and he/she lacks the reaction of attention. The whole complex of motor acts described above may be considered as the reflection of the appetitive phase of instinctive behavior of sleep. Thereafter, after a while, the subject's eyelashes slacken and the eyes gradually close. This highly coordinated motor event, which can be boldly regarded as the beginning of the consummatory phase of sleep, triggers a sharp change of EEG that is initially expressed mainly in the development of a regular alpha rhythm (Fig. 1B). Not infrequently this rhythm starts to develop as an episode that again depends on the change of extent of eye closure; but after closing them completely it may for a considerable time continue intermittently. Comparison by this parameter of different subjects of approximately one and the same age indicates that they may differ from each other as in the intensity of development of alpha rhythm, so in the duration of its occurrence, however, it ought to be noted that these parameters in one and the same subject are also dependent on the degree of desire to fall asleep. Against the baseline of the similar alpha rhythm before further deepening of the sleepy state in the subject, the conscious relation with the environment considerably attenuates as a result of which those comparatively weak sound stimuli, which before the closing of the eyes had been perceived, now become subthreshold. As to the stimuli which on this face may attract the attention of the subject result either in the reduction or complete blockade of alpha rhythm, that, apparently, again to some extent occurs in parallel with the development of tension of eyelashes and opening of the eyes. All this indicates that the alpha rhythm arising in the man ready to fall asleep in response to the closing of the eyes is the correlate not of the waking state as been regarded so far (Berger 1929; Beritoff and Vorobjev 1943; Lindsley 1952; Evans 1972; Cade and Coxhead 1979; Akerstedt and Gillberg 1990; Makeing and Jung 1995; Horne 1997; Aeschbach et al. 1999a, b; Cantero et al. 1999a, b, c; Ehrhart et al. 1999), but is a reflection of the beginning of the consummatory phase of sleep as an instinctive behavior that is apparently associated with pleasant perception resulting from the satisfaction of the intrinsic need (for more details on this point see subsequent publications). The data described above (Fig. 1A) clearly indicate that the EEG correlate of even passive wakefulness, proceeding in bed with the eyes open, but without activating the process of attention, is by no means the development of alpha rhythm, but the ordinary EEG desynchronization.
Further development of sleepy state is related approximately with such a sharp change in EEG pattern as is observed during closing of the eyes only with the distinction that it is associated already with the blockade of alpha rhythm. At this time, very often instead of a continuous alpha rhythm its fragments begin to develop and soon there occurs a total blockade of this rhythm. In accordance with the conventional classification it is considered that at this time wakefulness passes into a drowsy state or into stage 1 of sleep (Rechtschaffen and Kales 1968). Similar blockade of alpha rhythm, in our point of view, is likely to be related with that that, because of deepening of sleepy state, the brain ceases to realize the pleasant experience elicited by continuing satisfaction of intrinsic need for sleep. The deepening of sleepy state at this time is indicated by an appreciable enhancement of delta activity paralleled by fall in b2 activity in the EEG not only in comparison with wakefulness with the eyes open, but also with its preceding period of alpha rhythm development (Fig. 1C). Man (as well as the majority of mammalian species) is wakeful in order to establish intimate and adequate contacts with the environment that is largely achieved via visual analyzer. With closing the eyes, especially under the influence of development of a sleepy state, this contact gradually impairs what is the reason for a gradual transition from wakefulness to the state of sleep. Proceeding from this, the alpha rhythm appearing as a result of the eye closure cannot be regarded as the EEG correlate of wakefulness, as is the case at present.
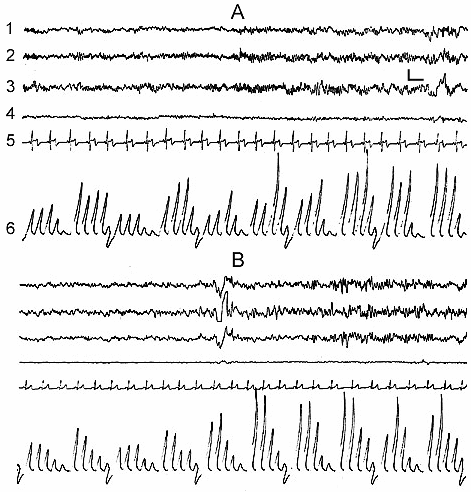
In the normal course of SWC the so-called sleep stage 1 in man is soon followed by the onset of stage 2, and then by the further formation of all its components (Fig. 1D) and in principle stage 1 may be considered as the beginning of stage 2. If for the onset of stage 2, apart from considerable enhancement of comparatively slow potentials in the EEG, characteristic is the appearance of spindle-like activity, then the already formed stage 2 is characterized by the development of the so-called K-complexes, which promote the enhancement of synchronization and transition of sleep to deeper stages (Fig. 1D). According to our data, K-complexes are not the EEG components of only sleep stage 2. They, although more seldom, but still may appear during sleep stage 3 and in a masked way even in stage 4. However, if during stage 2 the K-complexes mainly enhance the synchronization of slow waves of delta, theta and alpha range, then in stage 3 and 4 the generation of the same K-complexes in a particularly pronounced form may elicit an opposite effect, i.e. an appreciable suppression of delta and theta waves and generation of alpha rhythm on their face. In addition, it seems that, as K-complexes one should not consider only the evoked potentials, arising during synchronization, which have comparatively high and as it were, standard amplitude (Fig. 2B). Actually K-complexes may develop even in the form of threshold evoked potentials (Fig. 2A). Besides, the threshold K-complexes, as a rule, provoke synchronization in the EEG, while the high-amplitude K-complexes not infrequently show the tendency to desynchronization, not only during deep SWS (DSWS), as has just been indicated, but also during stage 2 of orthodoxal sleep (this will be described in more details in further publication).
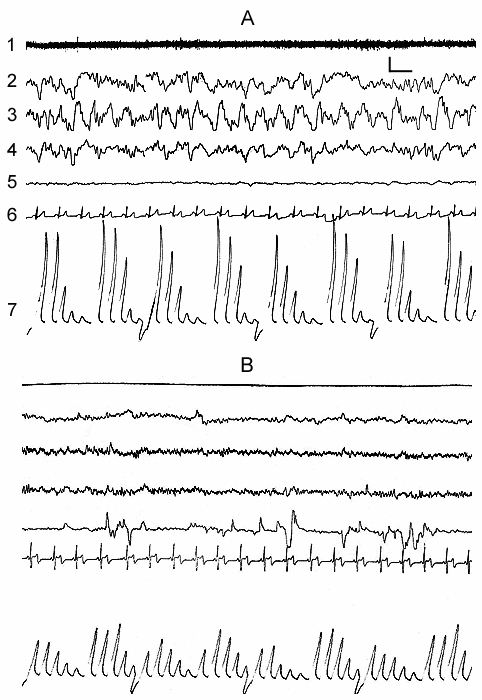
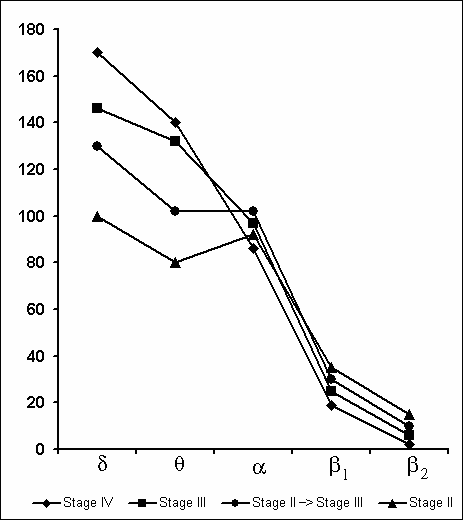
Synchronization of slow waves achieves its highest degree in stage 4, the so-called orthodoxal or SWS (Fig. 3A) which after a certain time gives way to the PS (Fig. 3B). The transition of sleep stage 4 to PS also occurs gradually, as if through the restoration of stage 3 and stage 2 of orthodoxal sleep. Yet these transient stages by their depth and physiological mechanisms, apparently, differ from stage 2 and stage 3 at orthodoxal sleep development. It should be mentioned here that the deepening of sleep state is a continuous process and determination of boundaries between the individual stages of orthodoxal sleep is rather conventional even by the EEG signs. This is indicated by the results of quantitative treatment of data with the use of the EEG spectral analysis in various stages and at the transition of one stage into another (Fig. 4). As is seen even in stage 2 the EEG shows the predominance of delta spectrum and at the same time the theta and alpha spectra, i.e. all the slow waves, are also well expressed, whereas the presentation in EEG of the spectrum of comparatively high-frequency oscillations (b1 and b2) appears to be weak. The transition of stage 2 to stage 3 is marked by the enhancement of all slow rhythms, especially of delta waves. The EEG analysis of stage 3 displays a selective increase of delta and theta rhythms that is especially well pronounced during EEG analysis of stage 4. Not denying the convenience and advantage of the presently adopted classification of sleep in general and of the orthodoxal phase of sleep in particular (its subdivision into four stages) for clinical purposes, in terms of polygraphic parameters (especially by EEG), from the point of neurobiology, more acceptable seems the subdivision of orthodoxal sleep in man, as well as in other mammals (Ursin 1968) into two stages - light SWS and DSWS.
The nocturnal sleep in man does not proceed as a smooth alternation of the orthodoxal and paradoxical phases of sleep. Very often, both in the young and old, during all stages of sleep may occur motor reactions associated largely with the change of the overall posture of the body or with the isolated movement of extremities. Such a motor activity even with no behavioral awakening causes sharp changes both of the EEG and somato-vegetative parameters. In particular, during movements the EEG shows the restoration of the pattern characteristic of the waking state (alongside with other slow waves the alpha constituent is also suppressed), the vegetative correlate of which is a significant increase in heart rate. If at this time there occurs no behavioral awakening and consequently the eyes remain closed, then shortly after this comparatively short fragment follows first the development of pronounced alpha rhythm (especially in the subject who at the beginning of falling asleep had a pronounced alpha rhythm), and then a regular restoration of all the rest stages of orthodoxal sleep and alternation of the phases of the cycle. It is necessary to mention that the phase of PS, as a rule, at its any length terminates with short EEG awakening after which the cycle starts anew. The moments of termination of PS phases with short episodes of awakening have been marked by us while plotting cyclograms, as to the motor reactions indicated above with no behavioral awakening, they, as a rule, were not taken into account. Although we think that with a more accurate analysis of the dynamics of various stages of sleep and considering the causal interrelationship between the phases of SWC they may yield additional information (for details see further publications).
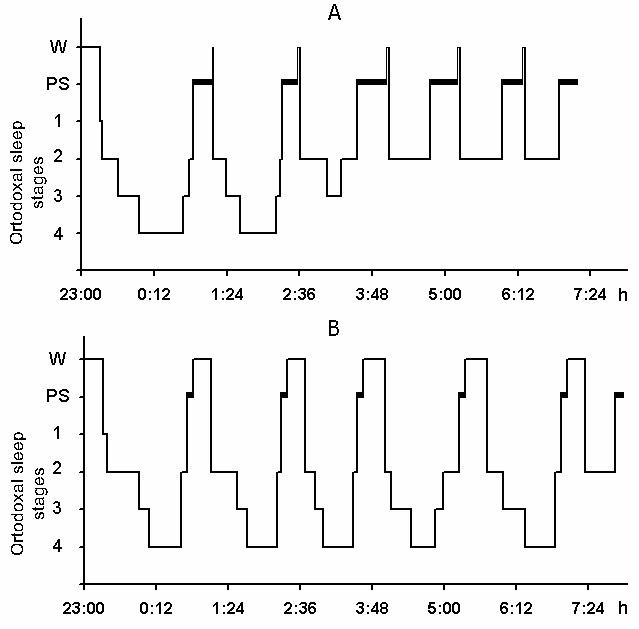
The cyclograms of a normal nocturnal sleep plotted by us on the basis of analysis of baseline polygraphic records both in young and elderly subjects keep basically within the standards established. Fig. 5A shows a cyclogram of one of the subjects (a 27-year old man) composed on the basis of analysis of baseline records before partial PSD. As is seen, during 3 hr and 40 min after the subject had made himself comfortable in bed and assumed a convenient pose of the body for sleep all stages of the orthodoxal sleep phase is realized and PS is triggered twice. After this DSWS does no longer develop as stage 4 and only once appears as stage 3 against which PS is triggered. The other phases of PS from 4 till 8 am are triggered from the level of stage 2 of orthodoxal sleep, after which the subject wakes up and announces that he is feeling very well and has had a good sleep.
2. Effect of partial PSD by means of replacing it with the equivalent in duration episodes of active wakefulness on the SWC structure in man.
As has been mentioned in the description of techniques, in the present series of experiments partial PSD was achieved by means of awakening of the subject 5-6 min after PS onset with a subsequent maintenance of active wakefulness for 10-15 minutes. The active wakefulness was maintenaned by making at this time the subject recollect and retell the content of the dream he had seen before waking up. During the inquiry the subject remained in bed in a lying position and having finished retelling had the possibility again to fall asleep calmly. In spite of this, after ceasing the contact with the observer, the examinee wakefulness condition may continue 10 minutes, that in most cases was caused by the reflections on details of private dreams' content. All the subjects readily underwent this procedure, as they well understood the task of the experiment and were interested in its normal course. After the night sleep and morning awakening the subjects were in good mood and their efficiency was on a normal level. As each subsequent night was considered to be the postdeprivation rehabilitation period the PSD experiments were repeated no earlier than after 48 hr.
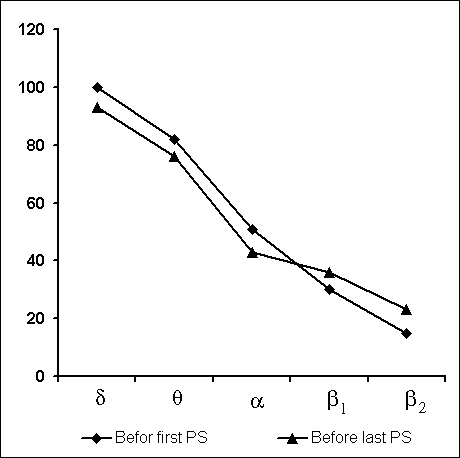
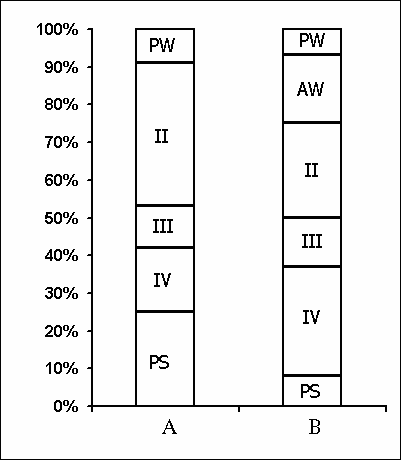
Under these conditions, the most interesting changes in SWC were observed namely in the course of partial PSD. The fact, first of all, is striking that during the application of the method in question there is no increase in the frequency of PS phases onset during PSD that is so characteristic of the PSD with instantaneous nonemotional awakening of the sleeping subject immediately after the PS onset not only in animals (Dement et al. 1967; Dement 1972; Oniani et al. 1988a, b; Camarini and Benedito 1997), but also in man (Dement 1960; Beersma et al. 1990; Endo et al.1998; Ferrara et al. 1999; Roth et al. 1999; Vu et al. 1999). Another no less important fact is that in the course of deprivation sharply alters the time distribution of the orthodoxal sleep stages. If in the baseline cycles of nocturnal sleep stage 3 and stage 4 are represented only during 2-3 hours after falling asleep (Fig. 5A), then during the partial PSD experiment the proper development of stage 3 and stage 4 is observed virtually throughout the entire nocturnal sleep (Fig. 5B). At this time after each awakening from PS and maintenance of active wakefulness for a definite time, all stages of orthodoxal sleep develop consecutively and the next transition from SWS to PS occurs also through the development of the so-called fragments of stage 3 and 2 (Fig. 5B). The results of quantitative treatment of data with the use of EEG spectral analysis of the constituent rhythms developing during stage 4 of orthodoxal sleep, preceding the first PS appearing a 1 hr and 30 min minute later of the sleep onset and the last PS, triggered via the transient stage again from the level of stage 4, 6 hr and 40 min after the onset of nocturnal sleep are presented in Fig. 6. As is seen, according to the expression of all the five rhythms, there is no statistically significant difference of stage 4 before the beginning of partial PSD and almost at the end of the experiment. It means that they do not differ from each other by the depth of sleep either. As to the ratio of different stages and phases of SWC during the deprivation night, it considerably alters in comparison with the normal cycle. This occurs first of all, because of the maintenance of rather long fragments of active wakefulness after the subject wakes up from PS, and secondly, because of the development of stage 3 and 4 of orthodoxal sleep after each of this fragment of wakefulness (Fig. 7). Naturally, all this leads to an increase of the total amount of DSWS.
Apart from no increase in the frequency of PS phases onset during PSD by means of partial replacement of these phases by adequate in duration episodes of wakefulness, the lack of accumulation of intrinsic need for the deprived phase is indicated also by analysis of the SWC postdeprivation records. Even in the first postdeprivation night the SWC of experimental subject does not differ from the baseline record in basic parameters, i.e. there is neither PS rebound according to the parameters of its amount and structure, nor any appreciable changes in the structure of the orthodoxal phase of sleep according to its distribution in separate stages.
As mentioned above, one of the aims of our experiments was to ascertain the relation between the EEG pattern and the character of dreams during PS. The thing is that during PS in man (Oglivie et al. 1982; Tyson et al. 1984; Akerstedt and Gillberg 1990; Dijk and Czeisler 1996; Werth et al. 1996, 1997; Cantero et al. 1999a, b, c), as well as in cat (Oniani et al. 1978) more or less prolonged fragments of alpha rhythm appear in the EEG. At this time other slow rhythms (of delta and theta range) remain suppressed. It may be assumed that during a prolonged phase of PS dreams seen in the presence of alpha rhythm in EEG would differ in pattern from those arising in its absence, i.e. during total EEG desynchronization. It appeared that actually there is a definite correlation between the EEG pattern and the content of dreams seen in PS. Although this issue merits further study, at this point we can say that dreams proceeding against alpha activity in PS are quieter and more pleasant than those seen in the absence of alpha rhythm in the EEG.
From the psychophysiological position the phenomenon of self-arousal observed by us during partial PSD with the awakening of the subject may be considered as more evident and reliable fact. The thing is that two subjects (by the way in both of them alpha rhythm was present in the EEG both at the beginning of falling asleep, as well as following the spontaneous motor acts occurring during different stages of sleep) after certain number of awakening from PS started to wake up by themselves and told the content of their dreams. As the reason for self-arousal they name recognition of the fact that they see a dream and understand that they are obliged to retell it to the experimenter; in this case we have to do with an obvious example of presently well known lucid dreaming (see LaBerge and Rheingold 1996) and the elaborated habit of self-arousal.
Aeschbach, D., Matthews, J., Postolache, T., Jackson, M., Giesen, H., Wehr, T. Two circadian rhythms in the human electroencephalogram during wakefulness. Am J. Physiol., 1999a, 277: R1771-R1779.
Aeschbach, D., Matthews, J., Postolache, T., Sher, L., Giesen, H., Jackson, M., Wehr, T. EEG theta / low alpha activity (5.29 - 9.0 Hz) during wakefulness is higher in short sleepers than in long sleepers. Sleep Res. Online, 1999b, 2(supplement 1): 514.
Akerstedt, T., Gillberg, M. Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 1990, 52: 29-37.
Akhvlediani, G., Oniani, T., Chikvaidze, V. The effect of some monoamine oxidase inhibitors on the sleep-wakefulness cycle of the cat. In: T. Oniani (Ed.), Neurobiology of Sleep-Wakefulness Cycle, Metsniereba, Tbilisi, 1988: 423-433.
Beersma, D., Dijk, D., Blok, C., Everhardus, I. REM sleep deprivation during 5 hours leads to an immediate REM sleep rebound and to suppression of non-REM sleep intensity. Electroencephalogr. Clin. Neurophysiol., 1990, 76: 114-122.
Beritoff, J., Vorobjev, J. On the origin of the facilitating action on alpha-waves in man, caused by closing the eyes. In: J.S. Beritoff (Ed.), Transactions of the J. Beritashvili Physiological Institute, Tbilisi, The Georgian SSR, 1943, 5: 369-387, (in Russian).
Berger, H. Uber das elektenkephalogramm des menschen. Arch. Psychiat., 1929, 87: 527-570.
Bodosi, B., Obal, Jr., Gardi, J., Fang, J., Krueger, J. Hypophysectomy and antiserum against prolactin block ether stress-induced REM sleep. Sleep Res. Online, 1999, 2(supplement 1): 174.
Cade, C., Coxhead, N. The awakened mind. Element Books, Longmead, Great Britain, 1979.
Camarini, R., Benedito, M. Rapid eye movement (REM) sleep deprivation reduces rat frontal cortex acetylcholinesterase activity. Braz. J. Med. Biol. Res., 1997, 30: 641-647.
Cantero, J., Atienza, M., Salas, R. EEG coherence pattern of alpha activity in three different brain activation states. Sleep Res. Online, 1999a, 2(supplement 1): 19.
Cantero, J., Atienza, M., Gomez, C., Salas, R. Spectral structure and brain mapping of human alpha activities in different arousal states. Neuropsychobiology, 1999b, 29: 110-116.
Cantero, J., Atienza, M., Salas, R., Gomez, C. Brain spatial microstates of human spontaneous alpha activity in relaxed wakefulness, drowsiness period and REM sleep. Brain Topography, 1999c, 11: 4.
Darchia, N., Oniani, T., Gvilia, I., Maisuradze, L., Lortkipanidze, N., Mgaloblishvili, M., Chidjavadze, E. Analysis of competitive interrelationship of wakefulness and paradoxical sleep using two different methods of paradoxical sleep deprivation. Sleep Res. Online, 1999, 2(supplement 1): 521.
Dement, W. The effect of dream deprivation. Science, 1960, 131: 1705-1707.
Dement, W. Sleep deprivation and the organization of the behavioral states. In: C. Clemente, D. Purpura and F. Mayer (Eds.), Sleep and Maturing Nervous System, Acad. Press. New York, London, 1972: 319-361.
Dement, W., Henry, P., Cohen, H., Fergusson, J. Studies of the effect of REM deprivation in humans and in animals. In: S. Kety, E. Evarts, and H. Williams (Eds.), Sleep and Altered States of Consciousness, Williams and Wilkins, Baltimore, Maryland, 1967: 456-468.
Dijk, D, Brunner, D, Breesma, D., Borbely A. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep, 1990, 13: 430-440.
Dijk, D. and Czeisler, C. An endogenous circadian rhythm of EEG alpha activity in REM sleep. J. Sleep Res., 1996, 5: 1-50.
Dusan-Peyrethon, I. and Jouvet, M. Supression elective du sommeil paradoxal chez le chat par a methyl Dopa. Comptes Rendus das Seances de la Sociate de Boil., 1968, 162: 116-118.
Ehrhart, L., Toussaint, M., Simon, C., Gronifier, C., Luthringer, R., Brandenberger, G. Dissociation of the relationship between EEG alpha activity and cardiac correlates during interrupted nocturnal sleep. Sleep Res. Online, 1999, 2(supplement 1): 255.
Endo, T., Roth, C., Landolt, P., Werth, E., Aeschbach, D., Achermann, P., Borbely A. Selective REM sleep deprivation in humans: effect on sleep and sleep EEG. AJP - Regulatory, Integrative and Comparative Physiology. 1998, 274: 1186-1194.
Evans, F. Hypnosis and sleep: techniques for exploring cognitive activity during sleep. In: E. Fromm and R. Shor (Eds.), Hypnosis: Research Developments and Perspectives. Chicago: Aldine-Atherton, 1972.
Fergusson, J., Dement, W. Changes in intensity of REM sleep with deprivation. Psychophysiology, Baltimore, 1968, 4: 300-312.
Ferrara, M., De Gennaro, L., Bertini, M. Selective slow-wave sleep (SWS) deprivation and SWS rebound: do we need a fixed SWS amount per night? Sleep Res. Online, 1999, 2(1): 15-19.
Fishbein, W., Gutwein, B. Paradoxical sleep and memory storage processes. Behav. Biol., 1977, 19: 425-464.
Horne, J. The phenomenon of human sleep. Loughborough Sleep Research Centre. The Karger Gazzette, 1997, April.
Jouvet, D., Vimont, P., Delorme, F., Jouvet M. Etude de la privation selective de la phase paradoxale de sommeil le chat. C.R. Soc. Biol., Paris, 1964, 158: 756-759.
Jouvet, M. Behavioral and EEG effect of paradoxical sleep deprivation in the cat. Proc. Int. Congr. Physiology Sci., Tokio, 1965, 87: 344-355.
Jouvet, M. The neurophysiology of the states of sleep. Physiol. Rev., 1967, 47: 117-177.
Jovanovic, U. An experimental contribution to our knowledge of the phenomenology of sleep. In: Normal Sleep in Man. Hippokrates verlag Stuttgart, 1971: 254-315.
LaBerge, S. and Rheingold H. Exploring the world of lucid dreaming. "Sofia", Kiev, Transpersonal Institute, Moskow, 1996.
Lee, Ch., Kim, J., Choo, H., Han, J., Lee, S. Effects of amitriptyline on insomnia induced by chloramphenicol in freely-moving rats. Sleep Res. Online, 1999, 2(supplement 1): 174.
Lindsley, D. Psychological phenomenon and the electroenephalogram. EEG and Clin. Neurophysiol., 1952, 4: 443-450.
Making, S., Jung, T. Changes in alertness are a principal component of varianse in the EEG spectrum. Neuroreport, 1995, 7: 213-216.
Maisuradze, L., Lortkipanidze N., Oniani, T., Eliozishvili, M., Oniani, L., Mchedlidze O. Comparative studies of PGO-deprivation and REM-deprivation of paradoxical sleep on the structure of cat's sleep-wakefulness cycle. Neurobiology of Sleep-Wakefulness Cycle, 2001, 1(2): 57-63.
Maisuradze, L., Oniani, T., Lortkipanidze, N., Oniani, L., Eliozishvili, M. An organization of sleep-wakefulness cycle at the REM sleep deprivation. Proc. of the 3rd ASRS Congress, Thailand, Bangkok, 2000: A139.
Mallick, B. One of the functions of REM sleep is to maintain brain excitability possible cellular mechanisms of action. Proc. of the ASRS 3rd Congress, Thailand, Bangkok, 2000: A32.
Morden, B., Mitchell, G., Dement, W. Selective REM sleep deprivation and compensation phenomena in the rat. Brain Res., 1967, 5: 339-349.
Nachkebia, A. Functional Characteristic of Isolated Forebrain. 1989, Avtoreferat disertacii (in Russian).
Obal, F. Jr., Benedek, G., Lelkes, Z., Obal, F. Effects of acute and chronic treatment with amitriptyline on the sleep- wake activity of rats. Neuropharmacology, 1985, 24: 223-229.
Oglivie, R., Hunt, H., Tyson, D., Lucescu, M., Jeakins, R. Lucid dreaming and alpha activity: a preliminary report. Perceptual and Motor Skills, 1982, 55: 795-808.
Oniani, T. The correlation between emotional tension and EEG dynamics in wakefulness-sleep cycle. In: T.Oniani (Ed.), Neurophysiology of Emotion and Wakefulness-Sleep Cycle. Metsniereba, Tbilisi, 1976: 5-27.
Oniani, T. The Integrative Function of the Limbic System. Tbilisi, Metsniereba, 1980.
Oniani, T. Does paradoxical sleep deprivation disturb memory trace consolidation? Physiol. Behav., 1984, 33: 687-692.
Oniani, T. Neurophysiological analysis of paradoxical sleep deprivation. Mat. of Intern. Simp. "Neurobiology of Sleep-Wakefulness Cycle". Tbilisi, Metsniereba, 1986: 66-68.
Oniani, T., Adams, D., Molnar, P., Gvetadze, L., Manjavidze, Sh., Beradze, G., Mgaloblishvili, M., Korchinski, R., Varazashvili, P. Organization of unit activity of limbic structures in the sleep-wakefulness cycle. In: P. Kostyuk (Ed.), Studies of Mechanisms of Nervous Activity. Nauka, Moscow, 1984a: 215-218, (in Russian).
Oniani, T., Chijavadze, E., Maisuradze, L. Effect of slow-wave sleep partial deprivation on the sleep-wakefulness cycle. Sechenov Physiological Journal of the USSR, 1984b, 70,8:1142-1148.
Oniani, T., Lortkipanidze, N. Effect of paradoxical sleep deprivation on learning and memory. In: T. Oniani (Ed.), Neurophysiology of Motivation, Memory and Sleep-Wakefulness Cycle. Tbilisi, Metsniereba, 1985, 4: 136-213.
Oniani, T., Lortkipanidze, N., Maisuradze, L. Neurophysiological analysis of deprivation of paradoxical sleep. Abstracts of Scientific Conference: Topical Problems of Sleep Physiology and Pathology. Moscow, 1985: 64-66, (in Russian).
Oniani, T., Lortkipanidze, N., Maisuradze, L., Oniani, L. Neurophysiological analysis of the effects of paradoxical sleep selective deprivation. Neurophysiology, Kiev, 1988a, 20: 20-28, (in Russian).
Oniani, T., Lortkipanidze, N., Mgaloblishvili M., Maisuradze L., Oniani L., Babilodze M., Gvasalia M. Neurophysiological analysis of paradoxical sleep deprivation. In: T.Oniani (Ed.), Neurobiology of Sleep-Wakefulness Cycle. Tbilisi, Metsniereba, 1988 b: 19- 43.
Oniani, T., Maisuradze, L., Lortkipanidze, N., Mgaloblishvili, M., Chidzavadze, E., Oniani, L., Oniani, N., Babilodze M. Total paradoxical sleep deprivation though partial deprivation of slow wave sleep. Bulletin of the Georgian Academy of Sciences, Tbilisi, 2000, 161: 127-131.
Oniani T., Maisuradze, L., Lortkipanidze, N., Mgaloblishvili-Nemsadze, M., Oniani, L., Eliozishvili, M., Oniani, N. Is selective and complete paradoxical sleep deprivation possible? Neurobiology of Sleep-Wakefulness Cycle, 2001a, 1(1): 15-28.
Oniani, T., Mgaloblishvili, M., Gogichadze, M., Lortkipanidze, N., Maisuradze, L., Chijavadze, E., Manjavidze, SH., Oniani, N., Nachkebia N., Koridze, M., Kavkasidze, M. Dynamics of the level of excitability and activity of the brainstem and diencephalic ascending activating structures in the sleep-wakefulness cycle. Proc. Georgian Acad. Sci., Biol. Ser., 2001b, 27: 205-224.
Oniani, T., Molnar, P., Naneishvili, T. The Nature of Paradoxical Phase of Sleep. Neuroscience Translation. Fed. Amer. Soc. Exp. Biol., 1978, 15: 1.
Oswald, I. Pharmacology of sleep. In: O. Petre-Quadens and J.Schlag (Eds.), Basic Sleep Mechanisms. Acad. Press, New York, London, 1974: 297-302.
Pickworth, W., Sharpe, L., Nozak, M., Martin, W. Sleep suppression induced by intravenous and intraventricular infusions of methoxamine in the dog. Experiment. Neurol., 1977, 57: 999-1011.
Rechtschaffen, A., Gilliand, M., Bergmann, B., Winter, S. Physiological correlates of prolonged sleep deprivation in rats. Science, 1983, 221: 182-184.
Rechtschaffen, A., Kales, A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. National Institute of Health Publication, Government Printing Office, 1968, 204.
Rechtschaffen, A., Siegel, J. Sleep and dreaming. In: E.R. Kandel, J.H. Schwartz and T.M. Jessel (Eds.), Principles of Neuroscience. McGraw-Hill, New York, 2000: 936-947.
Roth, C., Endo, T., Landolt, H., Werth, E., Werth, E., Borbely, A., Achermann, P. Selective REM deprivation: effect of frequent awakenings on sleep latency and intermittent sleep-time. Sleep Res. Online, 1999, 2(supplement 1): 552.
Sakai, K., Sastre J., Kanamori, N., Jouvet, M. State-Specific neurons in the ponto-medullary reticular formation with special reference to the postural atonia during paradoxical sleep in the cat. In: O. Pompeiano and C. Ajmone Marsan (Eds.), Brain Mechanisms and Perceptual Awareness. Raven Press, New York, 1981: 405-429.
Sallanon, M., Sanin, M., Buda, C., Jouvet M. Serotoninergic mechanism and sleep rebound. Brain Res., 1983, 268: 95-104.
Sampson, H. Deprivation of dreaming sleep by two methods. Arch. Gen. Psychiat., 1965, 13: 79-86.
Sucheki, D., Duarte Palma, B., Tufik, S. Sleep rebound in rats following sleep deprivation induced by the modified multiple platform method. Sleep Res. Online, 1999, 2(supplement 1): 559.
Tyson, P., Oglive, R., Hunt, H. Lucid, prelucid and nonlucid dreams related to the amount of EEG alpha activity during REM sleep. Psychophysiology, 1984, 21: 442-450.
Ursin, R. The two stages of slow wave sleep in the cat and their relation to REM sleep. Brain Res., 1968, 11: 347-356.
Van Hulzen, Z., Coenen, A. The pendulum technique for paradoxical sleep deprivation in rats. Physiol. Behav., 1980, 25: 807-811.
Vogel, G. A review of REM sleep deprivation. Arch. Gen. Psychiat., 1975, 32: 749-761.
Vogel, G., Roth, T., Gillin, J., Mendelson, W., Buffenstein, A. REM sleep and depression. In: T. Oniani (Ed.), Neurobiology of Sleep-Wakefulness Cycle. Metsniereba, Tbilisi, 1988: 187-215.
Vu, H., Roth, C., Mathis, J., Hurni, C., Achermann, P., Basseti, C. REM Sleep regulation in narcolepsy: effects of selective REM deprivation. Sleep Res. Online, 1999, 2(supplement 1): 463.
Werth, E., Achermann, P., Borbely A. Brain topography of the human sleep EEG: anteroposterior shifts of spectral power. Neuroreport, 1996, 8: 123-127.
Werth, E., Achermann, P., Borbely, A. Frontooccipital EEG power gradients in human sleep. J. Sleep Res., 1997, 6: 102-112.
Wyatt, R., Kupfer, D., Fram, D., Snyder, F. Prolonged drug- induced total REM sleep suppression in depressed patients. Psychophysiology, 1969, 6: 258-266.