1. Analysis of the SWC structure in baseline records.
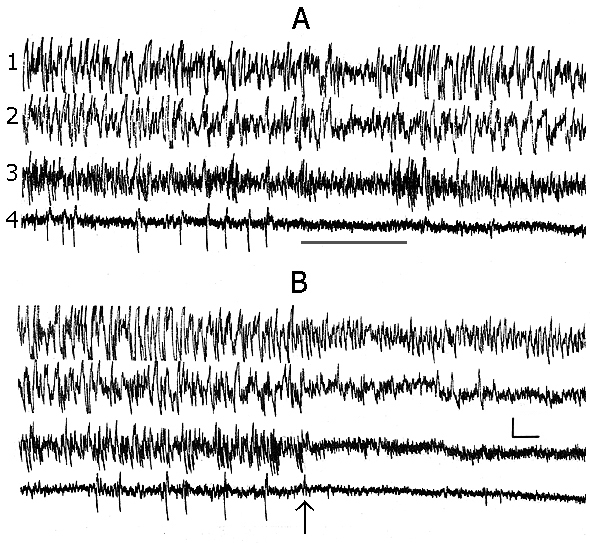
In baseline records, when the experimental animal has been already well habituated to the experimental chamber and electroencephalographic (EEG) manifestations of DSWS develop on the face of convenient for this position of the body (the cat is curled up, its head is lying so that the cervical muscle tone is virtually completely relieved) the appearing single PGO spikes do not always develop optimally for DSWS to pass regularly into PS. Perhaps, because of the enhancement of inhibition of the spinal cord motor reflexes (Pompeiano 1965), some hardly noticeable shifts occur in the animal's posture, leading to a weak activation of the brain, which in its turn slightly alters, in terms of EEG parameters, the depth of SWS (Fig. 1A), but is sufficient to block the generation of PGO spikes at the level of the midbrain pons. Similar situation, on the face of hardly seen fluctuation of EEG manifestation of SWS, may be repeated until proper transition of SWS to PS. It makes impression that the PGO spikes in these conditions are but one of the components of DSWS (see Dement 1972). They, in these cases too, are actually but the most cogent signs for the approachment of PS onset. This fact holds interest for the comprehension of delicate neurophisiological mechanisms of triggering and maintenance of PS. It is known that between the mesencephalic and diencephalic structures regulating the triggering and maintenance of wakefulness and SWS there is a reciprocal interrelationship (Bremer 1970; Moruzzi 1972). On the other hand, these structures of wakefulness via the inhibitory influence strictly control the triggering mechanisms of PS as well. Therefore during well developed DSWS as a result of intensive inhibition of the mesencephalic activating structures the PS triggering mechanisms are released from the inhibitory influence of the structures of wakefulness and trigger PS properly (Oniani 1977), the so-called third state of the brain (Jouvet 1962; Snyder 1963).
However, during DSWS and after the appearance of PGO spikes even a slight activation of mesodiencephalic structures of wakefulness (in this case as a result of change of the sleeping animal's body position) is sufficient to restore the inhibitory influence from the structures of wakefulness on those of PS. Analysis of this kind of the given fact leads to a speculative assumption that the brain structures triggering and maintaining SWS are not able to exert an inhibitory influence on the PS triggering mechanisms. Otherwise it would be hard to fancy even the fact that PS is being triggered namely on the face of DSWS, when the brain structures regulating the development of this phase work most intensively (Jouvet 1972). Here one should admit the existence of effective inhibitory influence on SWS regulating brain structures on the part of PS mechanisms. This would determine, as it were, unexpected transition of SWS to PS. It is likely that the assumption of Moruzzi (1972) concerning an unexpected appearance of PS was based namely on this, as though paradoxal fact. However, in our opinion, at more comprehensive analysis of the presently available facts there is nothing unexpected in that that the brain mechanisms regulating SWS namely during their maximal work stipulate triggering of PS, through inhibition of mesodiencephalic mechanisms of wakefulness, as well as further maintenance of this phase through the formation during its course of inner specific need for PS; this is clearly indicated by the below described results on PSD with different versions of nonpharmacological method.
Following a full adaptation of the animal to the experimental chamber, single PGO spikes appearing during DSWS, at the beginning, as a rule, get more frequent, then they are grouped, after which other parameters of PS are also triggered. It is namely at this stable course of SWC that the two versions of the classic nonpharmacological PSD method could be successfully compared.
2. Effect of REM-deprivation of PS on the SWC structure during deprivation and postdeprivation period.
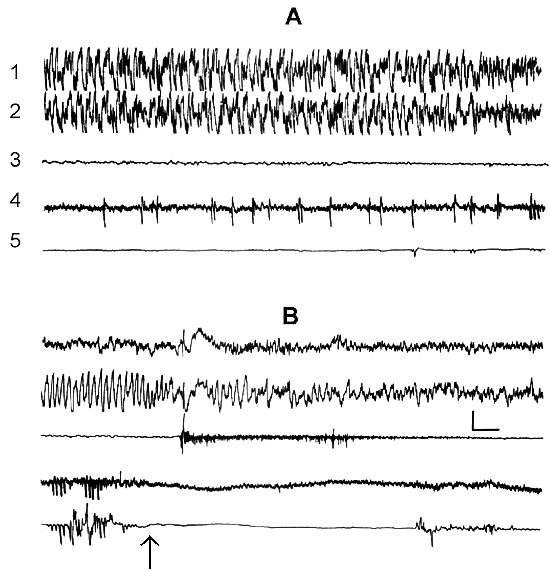
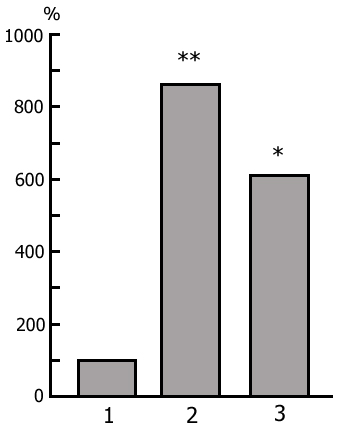
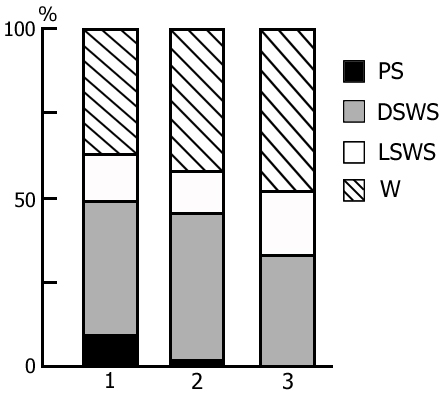
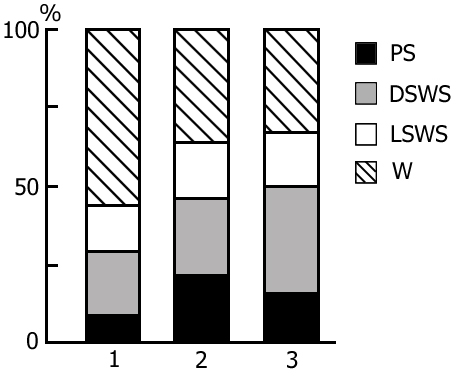
The transient stage from SWS to PS is known to be characterized by desynchronized electrical activity in the neocortex and by partial EEG desynchronization in the hippocampus (Oniani et al. 1978). At this stage no conspicuous REMs are observable. However, soon against the background of prolonged EEG desynchronization in the neocortex a regular theta rhythm starts to develop in the hippocampus. Characteristically, development of the hippocampal theta rhythm is correlated with the appearance of REMs and there occurs a full-fledged formation of the so-called REM phase of sleep (see Kleitman 1963). If after the appearance of REMs nonemotional awakening of the animal in response to electrical stimulation of posterior hypothalamus is effected, then against the background of continuous EEG desynchronization in the neocortex, both the PGO spikes and REMs vanish (Fig. 2B). On the face of nonemotional awakening the hippocampal theta rhythm is also considerably suppressed. After the animal's awakening from PS, short fragments of wakefulness are followed by SWS restoration and after a certain time PS appears in the layout as described above. Systematic awakening of the animal with the onset of each subsequent PS phase causes an increase in the frequency of applied arousing stimuli (Fig. 3, column 2). More frequent PS onset in similar experiments is considered to be a result of accumulation of the intracerebral specific need for the deprived phase of SWC in view of lack of development of full value PS in duration (Dement 1960). In the course of REM-deprivation, in parallel with fall out from the cycle of proper duration PS, marked changes occur in the other two, as it were, spared phases - wakefulness and SWS (Fig. 4, column 2). Apparently, total amount of both phases (and especially of SWS) is increased as compared to baseline (Fig. 4, column 1) because of PS elimination from the cycle.
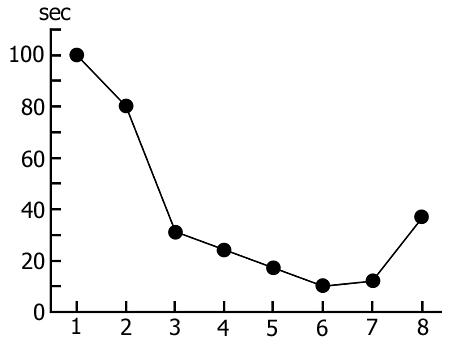
Accumulation of the specific inner need for PS during REM-deprivation has its impact on the rate of transition of SWS to PS. It is expressed in a gradual shortening of duration of that part of DSWS in which PGO spikes develop before turning into the full-fledged PS phase (Fig. 5). It is interesting to mention that if in the process of REM-deprivation of PS there arises a prolonged fragment of wakefulness that is naturally followed by SWS regularly passing into PS, then partially recovers duration of that part of DSWS during which develop PGO spikes (Fig. 5, the last circle). This fact indicates that a more or less prolonged fragment of wakefulness somehow delays the accumulation of the need for PS, or attenuates the level of its need already accumulated during deprivation. A conclusion can be drawn that accumulation of the PS need during REM-deprivation may affect also DSWS expressed in the shortening of its quota duration for PS triggering.
A more or less prolonged elimination of PS from the SWC through REM-deprivation exerts a significant influence also on the structure of cycle in the postdeprivation period that manifests itself in a marked increase of PS amount and respectively, a decrease of the share of wakefulness (Fig. 6, column 2), compared to the baseline SWC (Fig. 6, column 1). At this time the phase of SWS undergoes less pronounced alteration. Here particularly interesting seems the reciprocal interrelationship between the phases of wakefulness and PS in the SWC. Similar concurrence is apparently realized in the satisfaction of the inner need accumulated during REM-deprivation. It has been demonstrated earlier (Oniani et al. 1988a, 1988b, 2001) that replacement of PS phases in the SWC by the fragments of active wakefulness equivalent in duration causes the cessation of PS accumulation, as a result of which during this version of deprivation there is neither rise in frequency of PS onset during deprivation, nor its rebound in the postdeprivation period. This fact, too, indicates on the concurrent interrelationship between wakefulness and PS in the SWC in consuming the inner need formed in the brain during SWS.
3. Effect of PGO-deprivation of PS on the SWC structure.
As been indicated above, this version of nonpharmacological PSD, the instantaneous nonemotional awakening of the animal was produced immediately after the appearance of first single PGO spikes during DSWS (Fig. 1B) so that the SWS stage also suffered partial deprivation. It appeared that with the use of this version, the number of awakenings for a standard fraction of time necessary to prevent SWS transition into PS is tangibly lower (Fig. 3, column 3) than in the case of application of REM-deprivation (Fig. 3, column 2). Naturally, partial deprivation of DSWS during PGO-deprivation has a noticeable effect on the ratio of wakefulness and SWS during the procedure that is expressed in a substantial decrease of DSWS amount and an increase both of the phase of wakefulness and light SWS (Fig. 3, column 3), compared to the respective data obtained at REM-deprivation (Fig. 3, column 2). As regards the postdeprivation ratio of various SWC phases, in this aspect, too, the results obtained at PGO-deprivation of PS, tangibly differ from those obtained at REM-deprivation. First, although in this case too significant PS rebound is available in the postdeprivation SWC, but quantitatively it is markedly less (Fig. 3, column 3) than the PS rebound after cessation of REM-deprivation (Fig. 3, column 2). Besides, following PGO-deprivation in contrast to the data obtained in the version of REM-deprivation, there is a significant rebound also of DSWS in the postdeprivation SWC.
This fact can be readily explained by that that at PGO-deprivation there occurs also a partial deprivation of DSWS and thereby a more or less intensive accumulation of inner need for this phase too. On the other hand, the partial DSWS deprivation must retard the formation of the specific PS need, what, naturally, may affect the extent of rebound of this phase in the postdeprivation period.
Aserinsky, E., Kleitman, N. Regularly occurring periods of eye motility and concomitant phenomena during sleep. Science, 1953, 118, 3062: 273-274.
Bremer, F. Preoptic hypnogenic focus and mesencephalic reticular formation. Brain Res., 1970, 21: 132.
Dement, W. The occurrence of low voltage, fast electroencephalogram patterns during behavioral sleep in the cat. Electroenceph. Clin. Neurophysiol., 1958, 10, 2: 291-296.
Dement, W. The effect of dream deprivation. Science, 1960, 131, 3415: 1705-1707.
Dement, W. Sleep deprivation and the organization of the behavioral states. In: C. Clemente, D. Purpura and F. Mayer (Eds.), Sleep and Maturing Nervous System. Acad. Press. New York and London, 1972: 319-361.
Dement, W., Henry, P., Cohen, H., Fergusson, J. Studies on the effect of REM deprivation in humans and in animals. In: S.S. Kety, E.V. Evarts, and H.L. Williams (Eds.), Sleep and Altered States of Consciousness. Williams & Wilkins, Baltimore, Maryland, 1967: 456-486.
Fishbein, W. Disruptive effects of rapid eye movement sleep deprivation on long-term memory. Physiol. Behav., 1970, 6, 4: 279-282.
Jasper, H., Ajmone-Marsan, C. A Stereotaxic Atlas of the Diencephalon of the Cat. Montreal Neurological Institute of McGill University. Ottawa, Canada, The National Research Council of Canada, 1954: 68.
Jouvet, D., Vimont, P., Delorme, F., Jouvet, M. Etude de la privation selective de la phase paradoxale de sommeil le chat. C.R. Soc. Biol., Paris, 1964, 158, 4: 756-759.
Jouvet, M. Recherches sur les structures nerveauses et mechanismes responsables des differentes phases du sommeil physiologique. Arch. Ital. Biol., 1962, 100, 2: 125-206.
Jouvet, M. Paradoxical Sleep. A study of its nature and mechanisms. Progr. Brain Res., 1965, 18, 257: 20-57.
Jouvet, M. The role of monoamines and acetylcholine containing neurons in the regulation of the sleep-waking cycle. Ergebn. Physiol., 1972, 64: 166-307.
Jouvet, M., Michel, F. Correlations l'electromyographiques du sommeil chez la chat decortique et mesencephalique chronique. C.R. Soc. Biol., Paris, 1959: 422-425.
Kleitman, N. Sleep and Wakefulness. The University of Chicago Press. Chicago and London, 1963: 551.
Moruzzi, G. The sleep-waking cycle. Ergebn. Physiol., 1972, 64, 1: 64-165.
Oniani, T. On the functional significance of sleep. Acta Neurobiol. Exp., 1977, 37, 4: 223-246.
Oniani, T. Does paradoxical sleep deprivation disturb memory trace consolidation? Physiol. Behav., 1984, 33, 5: 687-692.
Oniani, T., Lortkipanidze, N. Effect of paradoxical sleep deprivation on the learning and memory. In: T. Oniani (Ed.), Neurophysiology of Motivation, Memory and Sleep-Wakefulness Cycle. Metsniereba, Tbilisi, 1985, IV: 136-214.
Oniani, T., Chidjavadze, E., Maisuradze, L. Effect of slow-wave sleep partial deprivation on the sleep-wakefulness cycle. I. Sechenov, Physiol. J. USSR, 1984, 70, 8: 1142-1148 (in Russian).
Oniani, T., Molnar, P., Naneishvili, T. The nature of paradoxical phase of sleep. Neuroscience Translation. Fed. Amer. Soc. Exp. Biol., 1978, 15, 1: 178.
Oniani, T., Lortkipanidze, N., Maisuradze, L., Oniani, L. Neurophysiological analysis of the effects of paradoxical sleep selective deprivation. Neurophysiology, Kiev, 1988a, 20, 1: 20-28 (in Russian).
Oniani, T., Lortkipanidze, N., Mgaloblishvili, M., Maisuradze, L., Oniani, L., Babilodze, M., Gvasalia, M. Neurophysiological analysis of paradoxical sleep deprivation. In: T. Oniani (Ed.), Neurobiology of Sleep-Wakefulness Cycle. Metsniereba, Tbilisi, 1988b: 19-43.
Oniani, T., Maisuradze, L., Lortkipanidze, N., Mgaloblishvili, M., Chidjavadze, E., Oniani, L., Oniani, N., Babilodze, M. Total paradoxical sleep deprivation through partial deprivation of slow wave sleep. Bulletin of the Georgian Academy of Sciences, Tbilisi, 2000, 161, 1: 127-131.
Oniani, T., Maisuradze, L., Lortkipanidze, N., Mgaloblishvili-Nemsadze, M., Oniani, L., Eliozishvili, M., Oniani, N. Is selective and complete paradoxical sleep deprivation possible? Neurobiology of Sleep-Wakefulness Cycle, 2001, 1, 1: 15-28.
Pompeiano, O. Ascending and descending influences of somatic afferent volleys in unrestrained cats: supraspinal inhibitory control of spinal reflexes during natural and reflexly induced sleep. In: M.Jouvet (Ed.), Aspects Anatomo-Functionneles de la Physiologie du Sommeil. Centre Nats. Res., Paris, 1965: 306.
Rechtschaffen, A., Gilliand, M., Bergmann, B., Winter, J. Physiological correlates of prolonged sleep deprivation in rats. Sciense, 1983, 221, 4606: 182-184.
Snyder, F. The new biology of dreaming. Arch. Gen. Psychiat., 1963, 8, 4: 361-391.
Van Hulzen, Z., Coenen, A. The pendulum technique for paradoxical sleep deprivation in rats. Physiol. Behav., 1980, 25, 5: 821-826.