I. Effect of sleep deprivation on the acquisition of instrumental alimentary conditioned reflexes in cats.
PSD was effected in two ways: (a) by awakening at the onset of PS and (b) by the water tank procedure according to Jouvet [95], with the animal being placed on a small pedestal over water in a special chamber. To study the effect of selective PSD on learning and memory, as well as on other manifestations of the cerebral activity, the former procedure, which does not lead to a substantial change of the animal's emotionality, is more appropriate [156]. However, in the studies undertaken in this direction the latter procedure has to date been given preference; hence, for the purpose of comparison, we have had to resort to it.
1. Effect of selective deprivation of PS by non-emotional awakening. Experiments with the former procedure of selective deprivation of PS were conducted in the following way: from 10 to 11 a.m. experiments, based on sound discrimination, were carried out with acquisition of instrumental alimentary conditioned reflexes to two feeders. Each conditioned signal (tone of 200 Hz and clicks), followed by food reinforcement, was repeated 10 times. After one session, from 11 a.m. the cats were given freedom in the experimental chamber. Satiated cats soon fell asleep and the sleep-wakefulness cycle was recorded for 6 h, from 11 a.m. to 5 p.m. However, at the onset of PS the animals were awakened by pulling a string fastened to the forepaw, shortly after this the slow wave phase was restored. After 5 p.m. the cats were taken to the vivarium where they could sleep normally. This type of procedure lasted for a few days until a solid acquisition of instrumental alimentary conditioned reflexes based on sound discrimination was reached. It turned out that selective PSD by awakening during 6 h after each learning session had no marked effect on the acquistion of instrumental alimentary conditioned reflexes. In the experimental animals, as well as in the controls, 100% discrimination of the conditioned sounds is attained within 4 days with one and the same number of pairings of a conditioned signal with food reinforcement.
Delay in acquisition of instrumental alimentary reflexes is not observed either in the case when PSD by non-emotional awakening was performed throughout the learning session intertrial period (Fig. 1). No marked changes were observed either in the emotional behavior of the experimental animals; they do not develop hyperphagia and hypersexuality described in the literature [38, 54, 90, 141, 198, 216] as a result of PSD by the water tank procedure.
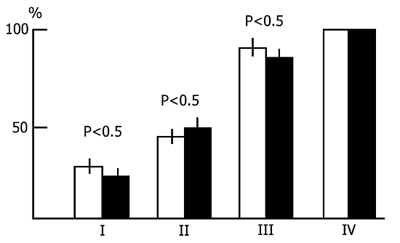
2. Effect of PSD by the water tank procedure on the acquisition of instrumental alimentary conditioned reflexes. In a series of experiments in which selective deprivation of PS was effected according to Jouvet [95] the sessions of acquisition of instrumental alimentary conditioned reflexes were held in the interval between 10 and 11 a. m. Following the sessions, till 5 p. m., the cats were placed in the chamber for water tank deprivation of PS. Neither did the use of PSD of this type reveal any difference between the experimental and control animals in the rate of acquisition of instrumental alimentary conditioned reflexes. In this case, too, a 100% discrimination of the conditioned sound signal was achieved in 3-5 days with the same mean number of pairings of conditioned and unconditioned stimuli in both groups of animals (Fig. 2).
A 6 h water tank PSD following the learning sessions does not alter significantly the general and emotional behavior of the animals. No change was found in the specific forms of motivation (hyperphagia, hypersexuality, etc.).
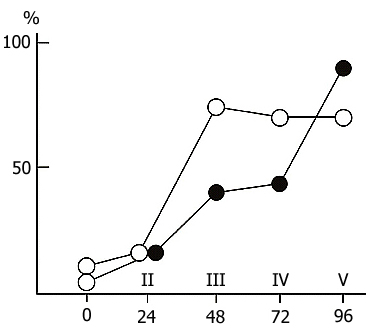
A different picture is observed if the sessions of acquisition of instrumental alimentary conditioned reflexes are run against the background of a more prolonged water tank PSD. In this case, first, the acquisition of conditioned signal discrimination is tangibly retarded and, second, with the continuation of PSD, there sets in a decline in the achieved level of conditioned signal discrimination. Fig. 3 shows the dynamics of discrimination between two conditioned stimuli in the case when the learning sessions are run during prolonged and continuous water tank PSD. The first session (10 pairings of each conditioned signal with food reinforcement) was run before PSD. The second and third sessions were against the background of 24 and 48 h deprivations, respectively. As is seen, by this time a fairly high level (80-85%) of discrimination of conditioned signals has been achieved. However, further continuation of PSD results in a sharp impairment of the discrimination of conditioned signals during the subsequent learning sessions. However, this must not be the result of an impairment of the memory mechanisms per se, since normal sleep restores the level of discrimination without training.
3. Effect of PSD on previously acquired instrumental alimentary reflexes. A special series of experiments was run to study the effect of water tank PSD and the retrieval of preliminarily acquired discrimination of conditioned signals to two feeders. It appeared that the preliminarily acquired discrimination of conditioned signals shows a great resistance in relation to such a factor: it is not disturbed even by a 10-day PSD. At the same time, dramatic changes are observed in the general behavior of the animals, resulting from prolonged and continuous PSD. Primarily it should be noted that the working ability of the experimental animals drops sharply, as a result of which there is a gradual decrease in the number of runs to the signaled feeders, the animals becoming languid and drowsy, the cardiac rhythm increasing, etc.
4. Effect of total sleep deprivation on the acquisition of instrumental alimentary reflexes. One series of experiments was devoted to the study of the effect of total sleep deprivation on the acquisition of instrumental alimentary reflexes to two feeders. Here, too; the learning sessions were run from 10 to 11 a. m.; after this the cats remained in the experimental chamber under the permanent observation of the experimenter, and at the onset of drowsiness were immediately awakened. Such a procedure quickly causes the cats to become emotionally excited and restless; they run to the corners of the chamber, looking for a quiet place to sleep. After 48 h total sleep deprivation the animals become languid, their working ability falls, and in the course of the learning sessions, after a few pairings of conditioned signals with food reinforcement, they cease to leave the starting place where they lie down and settle for sleep. Nevertheless, acquisition of instrumental alimentary reflexes with 100% sound discrimination in the experimental animals is achieved, on an average, as quickly as in the controls. The acquired instrumental alimentary reflexes and discrimination of conditioned signals are not disturbed even at a further continuation of total sleep deprivation.
5. Effect of induced hippocampal epileptiform discharges following learning sessions on the acquisition of instrumental alimentary reflexes. When assessing the PS role in the memory organization [129, 131, 224] special significance attaches to the fact that during this state there develops a progressive theta rhythm [88, 98, 165]. The occurrence of theta rhythm in its turn points to a high activity of the septo-hippocampal system [64, 65, 139, 176, 177, 183, 218] which is traditionally considered to be an important link in the organization of different memory stages [3, 129, 131, 217]. Proceeding from these facts and considerations we thought it interesting to study the effect of hippocampal epileptiform discharges, repeatedly evoked after each learning session, on the acquisition of instrumental alimentary reflexes. We proceeded from the experiments performed earlier in our laboratory [161], demonstrating that epileptiform discharges produced by direct electrical stimulation of the hippocampus resulted in the suppression of its hippocampal theta rhythm and in a delay of sleep onset after the cessation of the stimulation. Thus, using the given experimental design it is possible to study the effect of simultaneous abolishment of hippocampal theta rhythm and paradoxical sleep on learning.
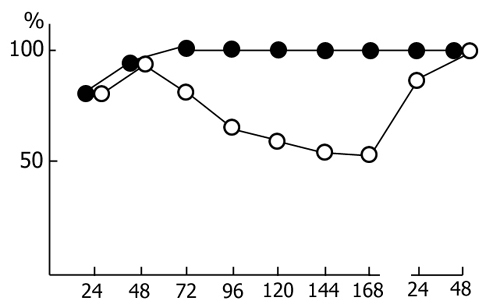
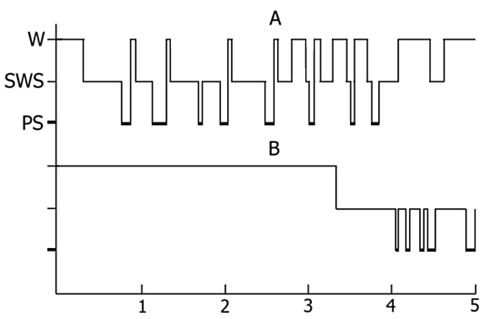
The experiments of this series were also standardized. As a rule, the acquisition of instrumental alimentary reflexes in cats occurred from 10 to 12 a. m. For two hours after the learning sessions repetitive epileptiform discharges were induced by direct electrical stimulation of the dorsal hippocampus, which involved only the hippocampus bilaterally or spread slightly to the neighbouring structures. In both cases the freezing reaction or faintly pronounced so-called "eating automatism" emerged as the behavioral correlates of the epileptiform discharges. For two hours it proved feasible to evoke epileptiform discharges 30 times on the average, with the intervals between the stimulations being long enough for recovery from electro-encephalographic (general depression of all rhythms of the hippocampus) and behavioral stimulation after-effects. During the indicated time suppression of the hippocampal theta rhythm increased progressively, persisting after the stimulation sessions were discontinued. With the cessation of hippocampal stimulation there occurred drastic changes in the sleep-wakefulness cycle, expressed primarily in a delay of the onset of sleep (Fig. 4). During wakefulness no pronounced theta rhythm was observed in the hippocampal electrical activity. After falling asleep the onset of paradoxical sleep was delayed and its structure changed considerably, which was manifested not only in the shortening of separate phases but also in a change of the EEG pattern. Over several phases the hippocampal theta rhythm was weakly expressed, subsequently recovering gradually and slowly. A complete normalization of the EEG pattern of PS occurred only on the second day.
Thus, in the above experiments, after each learning session, a disturbance of the normal functioning of the septo-hippocampal system was caused, on the one hand, and a 5-6 h PSD, on the other. Considering the view prevalent in the literature concerning the significance of the hippocampal theta rhythm [3, 129, 131, 166, 217, 225] and paradoxical sleep [see 58] in memory organization, our experiments seemed to provide the most unfavourable conditions for memory trace consolidation, which must underlie the acquisition of instrumental alimentary reflexes. In fact it turned out that the learning process in cats subjected to the indicated manipulations is slightly impaired only in the first learning sessions, while a 100% discrimination of conditioned signals is attained in the experimental group as readily in the case with the control group (Fig. 5). Thus, the present experiments do not support the view on the critical significance either of the high functioning of the septo-hippocampal system for learning, or of PS for memory trace consolidation.
II. Effect of PSD on the acquisition of an active avoidance reaction in rats.
1. Effect of preliminary PSD on the acquisition of an active avoidance reaction. In this series of experiments acquisition of active avoidance was effected in water tank PSD [140] animals. To this end, the duration of preliminary PSD was 24, 48 and 96 h. During PSD the control rats were placed in glass jars in which their motor activity was restricted. In the jars they could sleep normally without PSD.
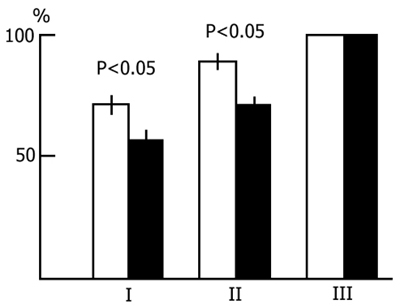
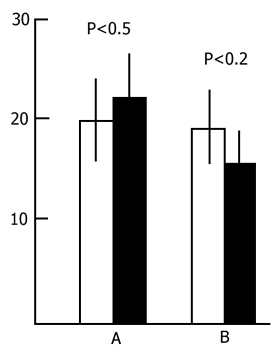
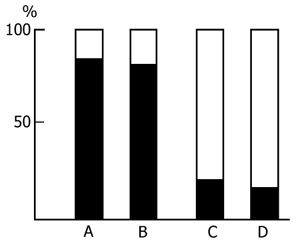
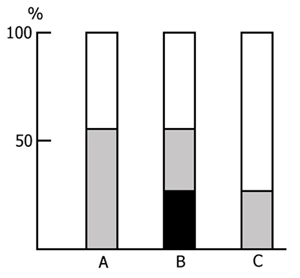
Preliminary - both 48 h and 96h - water tank PSD was found to have a facilitatory influence on the learning of an active avoidance test. Fig. 6 presents the results of statistical treatment of the data in this series of experiments. The light column shows the number of trials necessary to reach the criterion (9 correct responses out of 10 trials) of acquisition of active avoidance in the control animals, while the black column shows the same in PSD animals. As is seen, to reach the criterion of correct responses the control rats require much more trials than the experimental ones. What is more, the difference is statistically highly significant.
A comparison of the dynamics of acquisition of active avoidance both in the control and experimental animals shows that 96 h PSD facilitates the learning of an active avoidance test at all stages. The number of correct responses, i.e. conditioned avoidance reactions not reinforced with electrical stimulation, from the very beginning of the experiments increases faster in PSD rats than in control ones. This indicates that the experimental rats orientate better in the experimental situation and, as a result, learn to solve the task more readily.
2. Effect of preliminary water tank PSD on the retention and reproduction of an acquired active avoidance reaction. As demonstrated earlier, preliminary PSD by the water tank procedure considerably facilitates the acquisition of active avoidance in the rat. The effect of preliminary PSD by this technique on the retention and retrieval of an acquired response is also of some interest. To gain insight into this, experiments were carried out on two groups of rats. The control group animals were trained in active avoidance without preliminary PSD, whereas the experimental group was subjected to 96 h PSD. Then the animals of both groups were kept under the same con ditions and were afforded a normal course of sleep-wakefulness cycle. Thus, conditions were created under which, due to a rebound effect, the experimental animals had a considerably larger amount of PS in the sleep-wakefulness cycle during the interval between the learning and testing sessions, as compared with the control animals. If the amount of PS is of any importance in memory consolidation the experimental animals would show better fixation and reproduction of the learned response than controls.
Three days after a one-session learning the animals were tested for retention of the learned response. By the indicated time the number of pairings of the conditioned signal with unconditioned electrical stimulation, required for reaching the learning criterion (9 correct reactions out of 10 trials), was found to decrease drastically in both groups of animals (Fig. 7B) as compared with the number required in the first session (Fig. 7A). This points to the retention of a learned response in both groups of animals, though it is more highly-significant in the experimental group, which may be due to the smaller number of trials required for reaching the learning criterion in the first session. In the testing session, however, almost the same number of paired stimuli was required for the animals of both groups to reach the learning criterion (Fig. 7). This indicates, first, that an increase in the amount of PS coinciding with the consolidation phase does not lead to an improvement of conversion of short-term memory into long-term and, secondly, that facilitation of the acquisition of active avoidance in the learning session was due to the change of the general functional state of the nervous system resulting from PSD by the water tank procedure. These changes having levelled off by the second session 3 days later, the control and experimental animals found themselves under similar conditions in terms of the functional state of the nervous system, which resulted in the equalization of the number of paired stimuli required for reaching the criterion of acquisition of active avoidance.
3. Effect of post-trial PSD on retention of learned response. While training animals in active avoidance in one learning session to study the role of PS in memory trace consolidation it is advisable to perform PSD after learning rather than before it, as has often been done to date [58]. In the present case the memory trace consolidation phase will coincide with the absence of PS. It is natural to assume that if PS is necessary for memory trace consolidation, then its deprival just in the phase of trace fixation will have a negative effect on the conversion of short-term memory into the long-term form. To shed light on the problem 20 rats were train ed in active avoidance during one learning session. Then they were divided at random into two groups (10 rats in each). One group served as a control, whereas the animals of the other group were subjected to 72 h PSD by the water tank procedure. After that the animals of both groups were tested for retention of the acquired response.
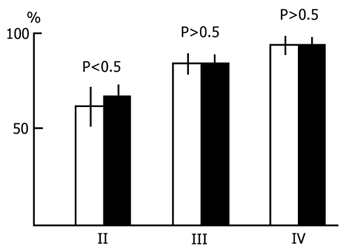
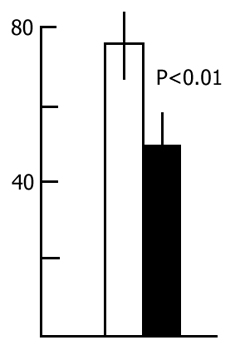
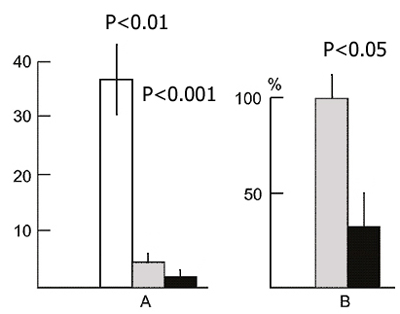
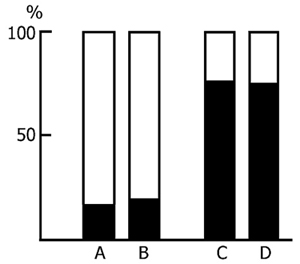
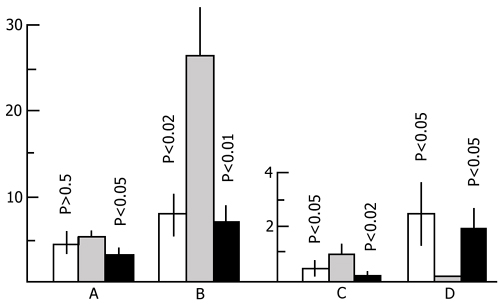
Both in the learning and testing sessions we determined the number of pairings of conditioned signal with unconditioned electrical stimulation required (1) for the acquisition of the first irregular avoidance responses, (2) for the acquisition of 2-3 consecutive correct responses out of 5 trials, and (3) for reaching the learning criterion (9 correct responses out of 10 trials). The results of statistical treatment of the data are presented in Fig. 6. It will be seen that for the acquisition of the first irregular response in the first session more than 10 pairings are required on an average (Fig. 8A, light bar); the acquisition of 2-3 correct responses out of 5 trials requires more than 20 pairings (Fig. 8B), and more than 40 pairings for reaching the learning criterion (Fig. 8C). 72 hours after the learning session the animals of both groups reach the above parameters with a much smaller number of pairings of the conditioned signal with unconditioned electrical stimulation. However, at all stages of acquisition of active avoidance the experimental animals have better indices than the controls. This is expressed in the following: in the testing session the experimental rats demonstrate avoidance in response to the first applications of the conditioned stimulation, whereas the control rats require a certain number of pairings (Fig. 8A). The smaller number of pairings required for the occurrence of 2-3 successive correct responses out of 5 trials (Fig. 8B) is also statistically significant. As to the number of pairings required for reaching the learning criterion it also tends to decrease, though the latter is not statistically significant (Fig. 8C).
It is clear from the above figure that the retroactive effect of PSD does not disturb memory trace consolidation and conversion of short-term memory into long-term after one training session in active avoidance. The fact that the experimental animals show facilitation of the retrieval of a learned response within 72 h as compared with the controls can be explained by the changes of the general state of the nervous system resulting from PSD by the water tank procedure. As was seen above, preliminary PSD by the given method considerably facilitates the acquisition of active avoidance during one learning session (Fig. 6). The same is the effect of PSD on the retrieval of a learned response in the active avoidance test, if the test is carried out against the background of deprivation.
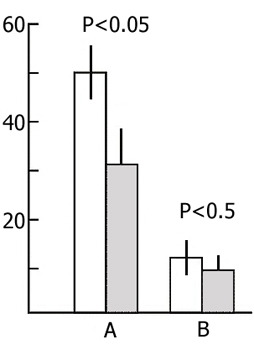
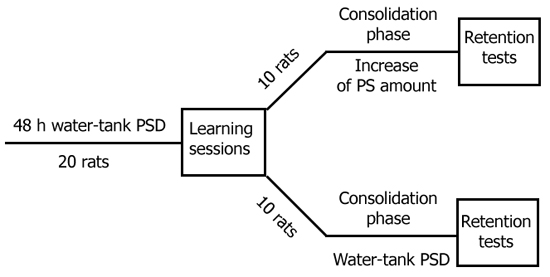
4. Effect of increased PS presence in the consolidation phase on retention of active avoidance response elaborated over one learning session. To clarify the significance of PS in memory trace consolidation it is advisible to create such experimental conditions which would permit a comparison between the data obtained in the case of PS absence in the consolidation phase with that of increased PS presence in this phase compared with the norm. Such a model is advisable because an increase in PS occurrence, resulting from preliminary learning, has often been reported in the literature to have a facilitating function in memory trace consolidation [27, 114, 123, 199, 227]. To this end we elaborated a special design of experiments (Fig. 9). It consisted in the following. The rats were first subjected to the water tank procedure. Then they were trained in active avoidance during one learning session (120 trials). The learning criterion was considered to be 9 correct responses out of 10. Then the rats were divided at random into two groups. Group I animals were subjected to a further 24 h or 48h PSD, whereas Group II animals were allowed to sleep normally. After 24 h or 48 h both groups were tested for retention of the learned response. Thus in the memory trace consolidation phase, i.e. in the interval between the learning and testing sessions, PS was absent in Group I animals, while in Group II it was represented to a higher extent as compared with the norm due to a rebound after previous 48 h deprivation. If PS is a necessary or facilitating factor in the memory trace consolidation phase the rats in which the presence of PS was increased for 24 h after the learning session should - at checking the retention of the habit - perform much better than those animals in which PS was absent during the same period of time. Group II animals should remember the learned response much better than those in Group I. But in fact there was an opposite picture. In the testing session the rats in both groups reached the learning criterion quicker than in the learning session (Fig. 10). However, the rats with additional 24 h or 48 h PSD reached the learning criterion after much fewer pairings of the conditioning signal with electrical stimulation (Fig. 10A) than did rats with increased PS presence. Fig. 10B shows that the difference in the number of trials required for reaching the learning criterion during the testing session both for rats with increased PS presence during the intersession period and rats with the absence of PS during that period is statistically significant.
The facts described above allow the following two conclusions to be drawn: (1) Increase in PS presence immediately after learning, i.e. in the memory trace consolidation phase, does not facilitate remembering of the learned response; (2) PSD by the water tank procedure during the consolidation phase has a facilitating effect on remembering in the active avoidance test, which is apparently due to changes caused by the stress situation attending this method of deprivation.
5. Post-trial effect of PSD on the dynamics of active avoidance behavior acquisition in distributed 1earning. As is known [see 24, 82, 137] the amnestic effect of different factors manifests itself in the course of learning (when memory trace consolidation is not completed), whereas the effect is absent when such factors are applied after the completion of learning. The same holds true in regard to the effect of PSD on the learning of active avoidance. Therefore, it is desirable to study the effect of PSD on the acquisition of active avoidance in distributed learning, when the acquired skills are gradually passed on from session to session. If PS is actually required for memory trace consolidation, then its deprivation in an intersession period should exert a most negative effect in terms of a delay in the acquisition of active avoidance. If PS plays a major role in the consolidation of memory traces, then post-trial PSD following each session should be most effective in the course of distributed learning. To this end, experiments were carried out in two groups of rats in the following manner: in the control group of 12 rats active avoidance behavior was elaborated for several days, so that in a one-day session a conditioned sound stimulus was only 20 times reinforced with unconditioned electrical stimulation delivered from the wire-mesh bottom of the chamber. In the intersession period the rats were placed in glass jars, which considerably restricted their locomotor activity. In the other group, with the same number of rats, acquisition of active avoidance behavior was effected in the same way, but between the learning sessions the rats were placed on pedestals in a special chamber for water tank PSD. In both groups determination was made of: 1) the mean number of trials required for the acquisition of a conditioned reaction of attention and alarm, 2) the mean number of trials necessary for the appearance of first irregular conditioned avoidance reactions, 3) the mean number of trials required for reaching the criterion (9 correct responses out of 10 trials) of the acquisition of an active avoidance reaction, and 4) the percentage of rats reaching the criterion in a daily session. Naturally, in the course of the sessions observation was also made of the changes in the general behavior of the control and experimental animals.
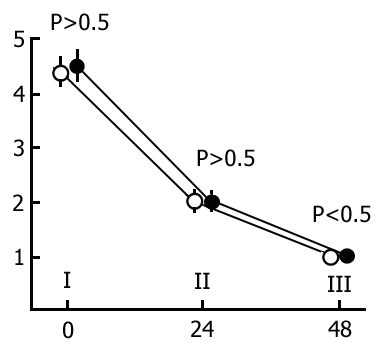
As expected, in the active avoidance test, a conditioned reaction of attention and alarm is the first to become elaborated in rats. Several pairings of the conditioned sound stimulus with electrical paw stimulation delivered from the wire-mesh bottom of the chamber proved enough to achieve this. Thus was this reaction elaborated in our experiments both in the control and experimental groups during the first learning session of active avoidance, i.e. before the start of the PSD procedure in experimental rats. As is seen in Fig. 11 for the acquisition of a conditioned reaction of attention and alarm both groups required almost the same mean number of trials. On the next day, in the course of the second session, i.e. following a 24 h PSD procedure, the mean number of trials for the appearance of a conditioned reaction of attention and alarm decreases in the same way in both groups. In the course of the third session, after the experimental rats have undergone 48 h PSD, a conditioned reaction of attention and alarm appears at the first application of the signal almost in all representatives of the control and experimental groups. From this it follows that PSD has no material effect on the acquisition of alarm reaction during the learning of a test of active avoidance.
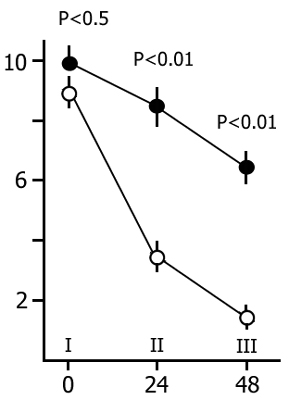
A different picture is observed when we have analysed the data on the acquisition of the first signs of an irregular active avoidance reaction. In some rats an irregular active avoidance reaction is elaborated in the first learning session of 20 trials. In the course of the second and third sessions the mean number of trials required for the appearance of a conditioned active avoidance reaction shows a stronger decrease in experimental rats than in the representatives of the control group (Fig. 12). This fact indicates that a 48 h PSD consider ably facilitates the acquisition of an active avoidance reaction.
During the subsequent sessions the number of rats reaching the criterion of learning in the active avoidance test increases progressively in both groups. At the beginning of the sessions active avoidance conditioning has an irregular character, while after a definite number of trials it starts to appear regularly, i.e. a learning criterion is reached. Naturally, the number of trials in each session for the irregular reactions of active avoidance to become regular decreases in the subsequent sessions. However, following the first 24 h PSD, the decrease in the mean number of trials, after which an irregular active avoidance reaction becomes regular, is more pronounced in the experimental group than in the control one.
As to the dynamics of the number of rats reaching the criterion of learning in a daily session, it also reveals the biphasic effect of prolonged PSD on the acquisition of active avoidance behavior. In Fig. 13 the light circles illustrate the percentage of animals reaching and failing to reach the criterion of learning the active avoidance test in the experimental group, while the dark circles indicate the same in the control group. As is evident, following 48 h deprivation, i.e. in the third learning session, the number of rats reaching the learning criterion sharply .increases as compared with the control group. In the fourth and fifth sessions, following 72 and 96 h PSD the number of rats reaching the learning criterion is not tangibly altered, while in the control group it is precisely in the last session that the percentage of animals reaching the learning criterion increases most abruptly. Such an abrupt rise in the percentage of animals reaching the learning criterion in the fifth session is to be explained by the accumulation of experience in the previous sessions. On the other hand, this indicates that in the course of prolonged PSD in the experimental animals some factor starts to have a negative effect on the learning process and the effect of this factor grows progressively. This factor is apparently dangerous for the life of the rats and accounts for a great percentage of lethality in the experimental group as compared with the control.
III. Effect of PSD on the learning of a passive avoidance test in rats.
1. Post-trial effect of 24 and 48 h PSD on memory in a passive avoidance test. Experiments were carried out in two series. In the first series study was made of the effect of 24 h PSD, and in the second, of 48h PSD on learning the passive avoidance test. In each series we had two groups of animals: control and experimental, each group consisting of 20 rats. The experimental procedure was similar to that used by Fishbein [see 58]. The experimental chamber had two compartments: light and dark. If the rats were placed in the light compartment, then after a definite period of time they penetrated into the dark one through the door. As soon as the rat entered the dark compartment the door was closed and a strong electrical stimulation was delivered from the metallic bottom of the chamber. After the cessation of stimulation the rats were immediately taken from the dark compartment to the vivarium. For 24 or 48 h conditions for a normal course of the sleep-wakefulness cycle were provided for the control group, while the experimental ones were placed in a special acrylic plastic chamber for a water tank PSD procedure. Then, the rats of both groups were again placed in the light compartment of the experimental chamber and again the latent period of entering the dark compartment was measured.
Before the commencement of the basic experiments we found it necessary to carry out a series of experiments to ascertain the effect of repeated placing of the rats in the experimental chamber on the latent periods, for it could be assumed that the previous experiment could somehow affect the speed of penetrating into the dark compartment from the light one. This series of experiments was carried out without application of electrical shocks in the dark compartment, though two groups of animals were compared. Representatives of one group were allowed a normal sleep-wakefulness cycle in the interval between the first and second placement in the experimental chamber, while in those of the other group the animals were subjected to 48h PSD procedure. Thus, in these experiments we could determine also the effect of PSD on the latency of the rat's penetration of the dark compartment from the light one. It is also seen that 48 h PSD did not have a considerable effect on the latencies in the given situation.
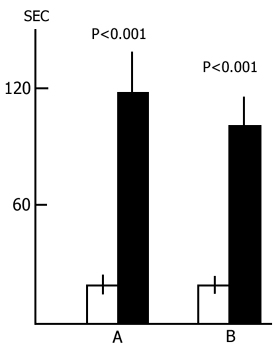
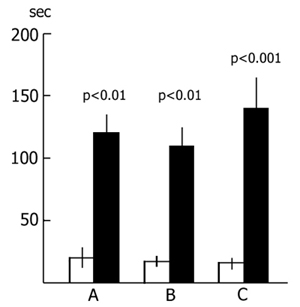
The results of a quantitative treatment of the data obtained in this series of experiments are presented in Fig. 14. The light columns designate the mean duration of latencies when the rats are first put into the experimental chamber, while the black columns designate the same parameter when they are repeatedly put there after 48 h without (Fig. 14A) and with (Fig. 14B) PSD. As is seen from the figure, the mean duration of the rat's penetration from the light section into the dark in both groups is about 20 sec, slightly altering in a repeated test. From this we concluded that repeated placing of the rats in the experimental chamber did not affect the latencies of penetration of the dark compartment from the light one. It became also evident that, in this situation, neither a 48 h PSD had any marked effect on the latencies.
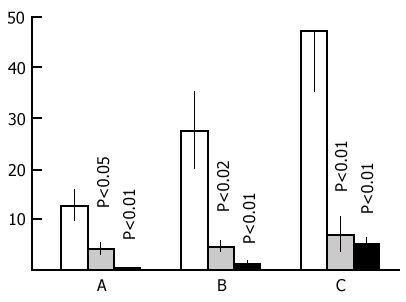
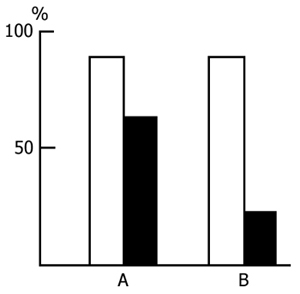
After ascertaining the effect of a repeated placing of the rats in the experimental chamber on the latency of entering the dark compartment we began the basic series of experiments. Application of an electrical shock in the dark compartment produces a sharp increase in the latency of entering it from the light compartment both within 24 and 48 h. As is known, this is considered to be a reflection of memory retention of a nociceptive electrical stimulation. In our experiments if a rat did not penetrate into the dark compartment during 5 min after it had been placed in the light one the observation of it was discontinued. It was found that, in the control group, most of the rats do not penetrate into the dark compartment. The longer the PSD the smaller is the number of rats in the experimental group penetrating into the dark compartment within 5 min. The percentage of rats, both in the control and in the experimental groups, entering and not entering the dark compartment within 5 min after their placement in the light compartment is presented in Fig. 15. It is seen that the number of such rats in the experimental group sharply decreases only following a 48 h PSD (Fig. 15B), while following a 24 h PSD (Fig. 15A) a fairly high percentage of experimental rats reaches the 5 min criterion, i.e. during this time they do not penetrate into the dark compartment from the light one. As to the control group, after both 24 h (Fig. 15A) and 48 h (Fig. 15B) application of an electrical shock almost the same number of rats reaches the 5 min criterion.
Judging by these data alone, one may conclude that PSD drastically impairs memory trace consolidation, as a result of which the experimental rats forget the situation where they received a nociceptive electrical shock, while the control ones remember the same event well. A similar conclusion is frequently reported in the literature [see 58 for ref]. Yet this conclusion can hardly be consistent, first, with the fact that following 24 h PSD (Fig. 15A) a high percentage of experimental rats show memory storage and fulfillment of the 5 min criterion. Second, a very interesting picture is observed (Fig. 16) if, following PSD, we compare the latencies of entering the dark compartment from the light one with the same parameters of the first test. In this figure the light columns show the mean duration of latencies of entering the dark compartment for all rats when they are first placed in the experimental chamber. It amounts to 20 sec. Black columns reflect the averaged values of latencies of penetration into the dark compartment for the rats of the experimental group which within 24 h (Fig. 16A) and 48 h (Fig. 16B) PSD after the electrical shock, do not reach the 5 min criterion. From this figure it is obvious that the latency of penetration into the dark compartment sharply increases in the rats of experimental groups that fail to reach the 5 min criterion.
The results of visual observations of the animal's behavior in the experimental chamber also indicate that the memory mechanisms are not disturbed under PSD influence in the passive avoidance test. The experimenter can easily notice that under the influence of the memory of electrical shock applied in the dark compartment the general behavior is altered in both groups when they are again placed in the light compartment. This is primarily reflected in the manifestation of fear. If, at the first placement in the experimental chamber, the rats penetrate into the dark compartment from the light one comparatively quickly and without any special signs of fear, within 24 h and 48 h of application of an electrical shock they begin to avoid the holes connecting the two compartments. After some time has elapsed since their placement in the light compartment the rats start exploring the holes, begin to slowly penetrate into the dark compartment, frequently withdraw in fear, some of them entering the dark compartment. Similar behavior is observed in the animals of both the control and experimental groups, being more pronounced in the latter. In both series of experiments (24 h and 48 h PSD) 40 rats were used in each of the control and experimental groups. When again placed in the light compartment the above behavior was observed only in 16 (40%) control rats. What is more, only 2 of them penetrated into the dark compartment. As to the experimental rats, similar behavior was observed only in 30 out of 40, i.e. in 75% of the total number. Of them 14 entered the dark compartment, while 16 remained in the light compartment more than 5 min. Thus, despite the obvious signs of fear, experimental rats seem to be more resolute in getting into the dark compartment from the light one, which is apparently due to the changes in the animals' emotional sphere under the influence of PSD.
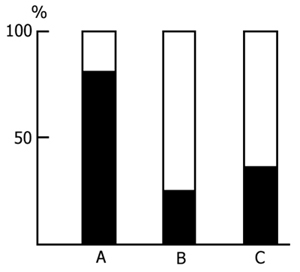
2. Post-trial effect of pairing of 24 h rest and 24h PSD on remembering in the passive avoidance test. In this series of experiments the effect of 24 h post-trial PSD in conjunction with prior postdeprivation 24 h rest on remembering in the passive avoidance test was studied. Open-field testing of the animals was also conducted. These experiments were prompted by two circumstances. 1. It is known that memory trace consolidation (if this is really necessary for the conversion of short-term memory into long-term) should develop most optimally in the first hours after perception of the information being processed [24, 28, 33, 46, 47, 82, 130, 180, 192, 212] and, it is reasonable to assume that a 24 h interval between the application of electrical shock and the beginning of PSD should be quite sufficient for the completion of the most optimal phase of consolidation. 2. If PS has indeed an important role in memory trace consolidation, then its 24 h deprivation after the application of electrical shock, in conjunction with a subsequent 24 h rest (by this time the changes in the animals emotional sphere must fade), may reveal the pure effect of PSD on remembering in the passive avoidance test. Experiments were carried out in three groups of animals (20 rats in each group). In the first group the effect of 24 h water tank PSD, in conjunction with a subsequent 24 h rest, was studied, while in the second group the situation was reversed: after the application of an electrical shock the animals were allowed a 24 h quiet rest, followed by a 24 h PSD, and shortly afterwards the retention of memory was checked. The third group served as a control: memory retention was examined 48 h after the application of an electrical shock. In all groups checked for memory retention 48 h after the application of electrical shock, the animals were found to fall into two subgroups. The first comprising the rats that within 5 min do not go from the light (safe) compartment into the dark (dangerous) one; the animals of the other subgroup failed to reach the 5 min criterion and penetrated into the dark compartment. However, the number of rats not penetrating into the dark compartment in the first group, in which the application of electrical shock was followed by 24 h PSD in conjunction with a subsequent 24 h rest (Fig. 17A), was considerably higher than in the second group (Fig. 17B), in which the application of electrical shock was followed by a 24 h quiet rest with a subsequent 24 h PSD. In the control group the percentage of rats penetrating and not penetrating into the dark compartment is approximately the same as in the first group (Fig. 17C). This clearly indicates that 24 h PSD immediately after the application of electrical shock has no material influence on remembering in the passive avoidance test. The data obtained in the second group of animals are of special interest. In spite of the fact that after the application of electrical shock they were allowed normal sleep for 24 h, subsequent 24 h PSD led to an increase in the percentage of rats penetrating into the dark compartment from the light one; those were the rats in which normal long-term memory does not seem to develop. However, analysis of the latencies of penetration from the light compartment into the dark one shows that memory is stored in all rats of the three groups.
3. Retroactive effect of 48h water tank PSD combined with 24 and 48 h rest. As shown by the above experiments, 48h water tank PSD exerts a maximally pronounced effect on the parameters both in the active avoidance test (Fig. 15B) and in the open field (Fig. 23). Bearing this in mind, it was of certain interest to study the effect of different combinations of 48 h water tank PSD with 48 h rest when the animals are afforded normal sleep. In this series of experiments, too, three groups of animals were compared. After the application of electrical shock the animals of the first group were allowed a quiet sleep for 48 h, followed by a 48 h PSD water tank procedure. Memory retention was checked within 96 h, immediately after the termination of PSD. In the second group, after the application of an electrical shock, first a 48 h PSD was given and then, before testing, the animals could sleep normally for 48 h. In the control group memory retention was checked 96 h after the application of electrical shock.
Here, too, in all the three groups the animals proved to fall into two categories: (a) those penetrating into the dark compartment within 5 min of their placement in the light one, and (b) those remaining in the light compartment. However, the percentage of rats penetrating and not penetrating into the dark compartment varies considerably in different groups (Fig. 18). As is seen, most of the animals of the first group which, after the application of electrical shock were permitted a 48 h quiet normal sleep and then subjected to 48 h PSD, did not reach the 5 min criterion and penetrated into the dark compartment (Fig. 18C), as well as during 96 h PSD after electroshock (Fig. 18D). In the second group, in which application of electrical shock was first followed by 48 h PSD and then given the opportunity of a 48 h quiet sleep, a reverse picture is observed (Fig. 18B). The majority of this group reaches the 5 min criterion, and a minor part enters the dark (dangerous) compartment. The ratio of the first and second halves in the control group is almost the same (Fig. 18A) as is in the second group.
The change of latencies of entering the dark (dangerous) compartment from the light (safe) one indicates memory retention also in those rats which fail to reach the 5 min criterion. The latency of entering at testing memory retention appears to be increased in all groups of animals in comparison with that before the application of electrical shock (Fig. 19).
4. Effect of 48h PSD in conjunction with a subsequent 3-5h rest on memory in the passive avoidance test. As has been shown above, a 24h rest entirely smoothes out the changes in the animal's emotional sphere produced by 48h PSD. It has been also ascertained that 48h PSD before rest has no marked effect on learning in the passive avoidance test. However, it could be thought that in such a combination of 24 h normal sleep with its paradoxical phases can compensate for the deficit in the memory trace consolidation process that could result from the preceding PSD. To check this assumption we conducted a special series of experiments in which 48 h PSD was followed by 3-5 h rest, in the course of which non-emotional quiet wakefulness was maintained without PSD.
After a 3-5 h rest under quiet wakefulness following 48 h PSD the majority of rats of the experimental group (Fig. 20C), like those of the control group (Fig. 20B), proved to succeed in reaching the 5 min criterion of being in the light compartment of the experimental chamber for the passive avoidance reaction to be checked. During 3-5 h rest, PSD-induced changes in the emotional sphere (determined by the open-field technique) fade to a considerable extent (Fig. 24).
Thus, this series of experiments clearly demonstrates the absence of the PSD effect on memory trace consolidation in the passive avoidance test.
5. Effect of proactive PSD on memory in a passive avoidance test. Fishbein [55] and Segales and Domino [190] observe that a 3-day PSD prior to an electric shock in the chamber for testing passive avoidance has no effect on the acquired behavior and short-term memory, yet it disturbs the conversion of short-term memory into long-term. The argument in favour of this is that mice to which electrical stimulation was applied after a 3-day PSD remembered it if memory was tested 1 h after the electrical shock, and failed to remember it after 24 h. Kruglikov et al. [108, 109] gave a 4-day PSD prior to electrical shock in a passive avoidance test and retention was checked 24 h after the shock. They report a diminution of latency for penetration into the dangerous compartment as compared to control ones, only in a part of the rats, while the other part reached the l0 min criterion well. In this partial decrease of latency the authors see not a disturbance of memory trace consolidation and conversion of short-term memory into long-term, but a disturbance of recurrent temporal connections. Thus, on this point, there are contradictions not only in facts but also in their interpretation.
Our experiments on rats were conducted as follows: immediately after 72h water tank PSD animals were placed in the light compartment and as soon as they penetrated into the dark compartment electrical shock was applied. The animals were then divided into three groups. In one group testing for retention of electrical shock was carried out after 10 sec, in another after 2h, and in the third after 24 h.
According to their behavior in the experimental chamber for the passive avoidance test, all the animals could be divided into three subgroups. The animals of the first subgroup, during 5 min do not even approach the door through which they could get into the dark compartment. Animals of the second subgroup approached and sniffed the door; some put their heads into the doorway but did not go into the dark compartment. A small number of rats, after having checked the situation at the door, did penetrate into the dark compartment. The percentage of these animals, tested after different periods of time of application of electrical shock, is illustrated in Fig. 21. As seen in the figure, when tested 10 min after electrical shock, for 5 min not a single animal enters the dark (dangerous) compartment from the light one (Fig. 21A). Yet most of them approach the door, as if checking the situation. 2 h after the application of electrical shock 27% of all animals in this group approach the door and enter the dark compartment, 30% go to the door but do not enter the dark compartment, and 43% do not even go to the door (Fig. 21B). 24 h after electrical shock application not a single animal penetrates into the dark compartment, and only 30% of the total number approaches the door (Fig. 21C). These findings obviously do not confirm the data of Fishbein [55], and Segales and Domino [190] on the preferential influence of proactive PSD on long-term memory, since the animals are in a better shape 24 h after electrical shock application (Fig. 21C) when tested for long-term memory than short-term memory (Fig. 21B). This is understandable if one takes into account the fact that changes in the animals' emotional sphere resulting from 72 h PSD can level off completely over a 24 h normal sleep-wakefulness cycle.
IV. Effect of PSD via water tank procedure on the rat's behavior in open field.
PSD by the water tank procedure is known to result in changes in the animal's motivational-emotional sphere [see 150, 220]. These changes are detectable in the form of development of hypersexuality, hyperaggressiveness [54, 70, 141, 198, 216], anxiety, hyperphagia [38, 90], as well as reduction of fear reaction [41, 70, 71, 72, 138, 150]. Of the known neurophysiological changes attending the PSD water tank procedure it is particularly interesting to note the enhanced excitability of the brain [31, 32, 167] and a respective decrease of the thresholds of induction of brain activation and epileptiform discharges. These changes should be borne in mind while studying the effect of PSD on learning and memory.
In our experiments signs of change in emotionality under the PSD water tank procedure were permanently observed in the chamber in which learning in the passive avoidance test was studied. This was expressed in the following: when the rats that had been placed in a water tank for PSD after an electrical shock were again put into the light (safe) compartment of the chamber they behaved in a more active way (walking about the compartment, rising, sniffing the floor and walls, etc.) than did those of the control group. However, in the latter group animals, as distinguished from the experimental rats, defecation and urination were observed. These changes were manifested with particular clarity in an open field study of behavior in both experimental and control groups.
According to the above-described experiments on the effect of water tank PSD on learning and memory in the passive avoidance test, the open field experiments may be divided into two parts:
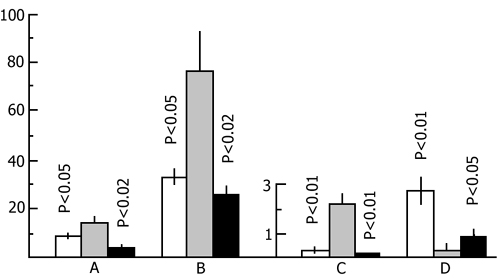
1. Effect of 24, and 48 h PSD in a water tank on the rat's open field behavior. In the literature a statement is found to the effect that 24h PSD via the water tank procedure allegedly has no material effect on the rat's emotionality [see 220]. This conclusion is based on a comparison of data obtained, on the one hand, from rats placed on small pedestals (experimental), and, on the other, from rats placed on comparatively large pedestals (control group). However, in our opinion, for a complete detection of changes in the emotional sphere under water tank PSD it is more reasonable to compare rats placed on small pedestals with their counterparts that have not been placed at all in the deprivation chamber where, irrespective of the pedestal size, here is a stressful situation. With this approach it appears that 24 h PSD has a tangible effect on the important parameters of the rat's open field behavior. In Fig. 19 light columns designate the averaged data for the control group on such parameters of open field behavior as the number of risings (Fig. 19A), number of squares crossed (Fig. 19B), that of entering the open field centre (Fig. 19C) and the number of defecations (Fig. 19D). All these parameters were measured over a 5 min stay in open field. The hatched columns designate relevant data obtained on rats which had been subjected to 24 h PSD in a water tank. It will be seen, that statistically highly significant changes are observed in such parameters as horizontal activity, and defecation. At the same time, enhancement of motor and exploratory activity as well as decrease of defecation in PS-deprived rats should point to the diminution of fear reaction. These data are in agreement with those observed in the experimental chamber when testing for memory retention. All this indicates that 24 h PSD in a water tank leads to changes in the animals emotional sphere.
With more prolonged PSD changes in the emotional sphere, as far as the above-mentioned parameters are concerned, develop still more vividly. Thus, for example, at 48 h PSD, in the experimental group (Fig. 20, dark columns) compared with the control rats (light columns) a statistically significant increase is observed not only in horizontal but also in vertical motor activity. In both cases experimental rats visit the centre of an open field more frequently than do the control ones (Fig. 20C). But the number of defecations is much higher in the latter than it is in the experimental group (Fig. 20D).
2. Effect of 24 h rest before and after water tank PSD on open field behavior in rats. As described above, in a special series of experiments we have studied the effect of differing-in-time combinations of PSD with rest, when the rats were allowed normal sleep for 24 h either before or after deprivation.
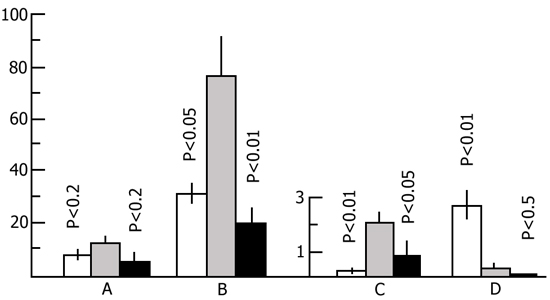
In one series of experiments in the animals of the first group electrical shock was followed by 24 h rest and then by 24 h PSD in a water tank, while in the second group an inverse combination of PSD and rest was tested. In the second series of experiments 24 h rest was combined with 48 h PSD. To estimate the role of the dynamics of emotional changes in this series we studied also the effect of 24 h rest following 24 h and 48 h PSD on open field behavior. 24 h rest following 24 h (Fig. 22) as well as 48 h (Fig. 23) PSD, was found to completely abolish the changes that could have occurred in the animals' emotional sphere.
It could be assumed that the leveling off of changes in the emotional sphere is due to the satisfaction of the need for PS over 24 h normal sleep. The more so if it is borne in mind that by this time there could have occurred a PS rebound. Yet, a subsequent series of experiments did not confirm this assumption. The changes in the emotional sphere resulting from 48 h PSD were found to disappear to a considerable extent during the subsequent 5 h rest as well, when the animals are artificially kept in a state of quiet wakefulness (Fig. 24).
This indicates, first, that emotional changes disappear not because of the restoration of normal sleep, but because of the elimination of the stressful situation, and secondly, the cause of development of emotional changes is not PSD per se, but the stressful situation existing in the water tank for the PSD procedure.
Over the last two decades the investigators' keen interest in the elucidation of the significance of PS in the regulation of learning and memory seems to have been prompted mainly by two circumstances: 1. As shown by neurophysiological studies, PS is an active state of the brain [36, 52, 53, 78, 88, 94, 98, 165, 194, 195] during which one can distinguish different levels of activity, as is the case in wakefulness [151, 152, 154]. In this regard it is especially worth noting that dreaming - always attracting much attention of the investigators concerned with the nature of consciousness - develops predominantly during PS [39, 40] and has a fairly pronounced emotional colouring. According to Dewan's hypothesis [42], the brain can be reprogrammed either spontaneously or in response to signals of sensory input, or to a current need of the animal. In the course of reprogramming the functional structure of the CNS may also alter. Proceeding from the belief that reprogramming requires a high level of emotionality, Dewan [42] assumes the predominant significance of PS in this process. By its recognition of the importance of a high level of activity of the CNS for information processing by the brain, this hypothesis seems to be in harmony with Lindsley's [122] activational theory of emotion and with Hebb's theory [67] on the significance of an optimally high activity of the brain in the resolution of key functions; nevertheless, this hypothesis is extremely one-sided. As a matter of fact, by its levels of brain activity and emotionality paradoxical sleep does not differ from the state of wakefulness. On the contrary, being a more important phase in the life of animals and man, wakefulness is characterized by a rich spectrum of levels of brain activity and emotionality, compared with PS. In this respect one may speak of the predominance of PS only over slow-wave sleep. However, there are no less convincing arguments in favour of the significance of SWS in the regulation of different steps of storage of memory traces [48, 49, 61, 72, 110, 111, 126, 226]. Moruzzi [143] apparently had precisely these arguments in mind when he pointed out the significance of SWS in the restoration of the synaptic apparatus involved in the regulation of memory.
2. Another cause of particular importance in the development of views regarding the role of PS in the regulation of memory must have been one of the popular propositions concerning the role of the hippocampus in the brain integrative activity. Following the clinical observations of Bekhterev [14], Penfield and Milner [174] and Milner [135, 136] the view has gained ground in neurobiology according to which the hippocampus plays an important role in the organization of those brain processes owing to which the so-called short-term or recent memory converts into long-term [3, 43, 50, 129, 130, 166, 212, 217, 225]. Notwithstanding the contradictory and hard-to-analyse data obtained in the animal experiments dealing with the role of the hippocampus in learning and memory processes, the above view is still widespread. In determining the functional significance of PS those studies have been of special importance in which memory trace consolidation or other steps of memory organization were related to the hippocampal theta rhythm [3, 50, 129, 130, 217]. It is in this aspect that special attention has been attracted by the fact that the hippocampal theta rhythm develops most intensively in PS [58, 89, 165]. If success in learning and memory trace consolidation processes is actually correlated with a pronounced hippocampal theta rhythm, as is often maintained, it is then reasonable to assert that in the course of PS, when the hippocampal theta rhythm is most intensive, the above processes, related to the organization of memory, should proceed at a high level. Since the pacemaker mechanism of the theta rhythm is localized in the septum [64, 65, 139, 176, 177, 183, 218], the septo-hippocampal system, which functions best in PS, is considered to be of almost crucial significance for the organization of memory [65, 218]. It should be noted, however, that the majority of facts derived, on the one hand from studies of the role of the septo-hippocampal system in the regulation of learning processes and memory, and, on the other, from studies of the significance of the hippocampal theta rhythm in the consolidation of memory traces, do not fit into the above - indicated scheme. It has been shown that neither lesion [see 80 for ref.] nor functional elimination [145] of the hippocampus have any marked effect on the rate of learning and memory retention. Neither has the view on the specific significance of the hippocampal theta rhythm for memory trace consolidation been confirmed, for lesion of the medial septal nucleus, leading to the disappearance of theta rhythm both in wakefulness and in PS, does not delay the learning process. In the experiments of Nachkebia and Oniani [145] distributed learning was studied by the method of acquisition of instrumental alimentary reflexes to two feeders, based on sound discrimination. At the same time after each daily session, 2 h hippocampal functional elimination was effected by evoking local bilateral epileptiform discharges. Such epileptiform discharges accounted for the hippocampal theta rhythm depression not only between the electrical stimulation trials, but also long after the cessation of the stimulation session (for 2 h and over). In these animals somnolence developed with long latency and, after the onset of sleep, the appearance of the first PS was retarded. Thus, in these experiments both hippocampal theta rhythm depression and 5-6 h PSD after each learning session were in evidence. Despite this, learning in experimental animals proceeded not a bit worse than it did in the controls. Thus, these findings do not confirm the importance of the hippocampus, hippocampal theta rhythm, and PS in the organization of learning and memory.
Thus the premises on which the authors based their hypothesis of the significance of PS in the organization of learning processes and memory are far from being convincing and cannot stand criticism. As to the factual material accumulated by the proponents of the informational theory, a critical examination reveals flaws in it too. Jenkins and Dallenbach [81], who discovered a facilitatory influence of sleep on the remembering of learned material, advanced an interference theory according to which during sleep, in contrast to wakefulness, there is no interference in the information received before sleep by some other information. Absence of interfering information should especially characterize not PS, but the slow wave phase of sleep against whose background there develop dreams that could act as an interfering factor [214]. As a matter of fact, Yaroush et al. [226], Fower et al. [61], Ekstrand et al. [49], Manov [126] have shown that the first half of an 8 h nocturnal sleep, when there prevails deep slow sleep, has a more facilitatory effect on the remembering of material than the second half, when the amount of stage 4 of slow sleep dramatically diminishes and PS prevails. Ekstrand et al. [49] explain this fact not from the position of the interference theory but in terms of the so-called decay theory according to which forgetting occurs due to the destructive effect of catabolic processes. As these processes are much lower during sleep than during wakefulness, the former acts as a factor facilitating remembering. Yet it is very difficult to reject the interfering effect of the processes occurring in the PS phases.
As indicated above, the brain activity in PS attains the level of active wakefulness [21, 23, 29, 52, 53, 78, 86, 151, 160]. Catabolic processes (if they can act as a factor of forgetting) should naturally occur more intensively in PS. Hence, viewed from this standpoint, PS may contribute to forgetting rather than to remembering the information received by the brain.
One of the arguments advanced in favour of the interfering function of PS is its parallel development with memory in the phylogeny of the animal kingdom [185]. Referring to the fact that PS and long-term memory [16] are lacking in fish and amphibians and first appear in birds and mammals [2, 10, 17, 18, 36, 73, 74, 91, 97, 102, 124, 184, 186 - 189, 204, 209 - 211, 222 - 224], excepting echidna [6], Rojas-Ramires and Drucker-Colin [185] suggest a functional interrelation of these two phenomena. At the same time, taking for granted the view that PS plays an extremely important role in memory trace consolidation via regulation of protein synthesis, they come to the following conclusion: "The incorporation of these findings to the consolidation on phylogeny of sleep and memory allows us to anticipate the following tentative interpretation: it is possible that the mechanisms of macromolecular synthesis determine, through phylogenetic evolution, a relationship between sleep and memory phenomena".
However, this obviously may not be the only conclusion. There is no doubt that due to qualitative changes in the internal environment there emerged a necessity in homoiothermal animals for a more complex and refined regulation of the organism's homeostasis in general and of brain homeostasis, in particular. Without this fine regulation, such an important function of the brain as memory could apparently not have evolved. The sleep-wakefulness cycle - in the form it is found in mammals - should be the result of an evolutionary perfection of the brain homeostasis which, in turn, is obligatory for the realization of all other functions of the CNS. In this aspect, to single out any separate phase of the sleep-wakefulness cycle seems unjustified, for the entire cycle acts as a functional unity in the regulation of homeostasis. Even a simple analysis of the Tab le adduced by the authors to illustrate the ratio of the various phases of the sleep-wakefulness cycle in mammals shows the untenability of singling out any one phase as being significant in the organization of memory. In this respect 24 h records of the sleep-wakefulness cycle in different species of mammals (Table I) are particularly informative. As seen from the Table, by the length of PS in 24 h records the opossum holds the first place, while man only the fifth. It can hardly be asserted that by the amount and quality of information processing in general, and of processes of remembering, in particular, the brain of the opossum is ahead of that of man or of any other species of mammals on a higher step of phylogenetic development. There is every reason for an opposite conclusion.
In defining the functions of various types of sleep the findings derived from the study of the formation of the sleep-wakefulness cycle in ontogenesis may prove helpful. It was precisely the fact that PS appears as early as in the intrauterine life of man and other mammals and prevails in early postnatal ontogenesis [60, 168, 175] that served as the basis of the theory of its ontogenetic function [182, 205]. At the same time, the fact that in the course of postnatal ontogenesis the enhancement of the informational work of the brain is paralleled by a decrease of the amount of PS, whereas the amount of slow wave sleep increases does not fit into the informational theory of sleep. Small wonder that the recognition of a functional relationship between the organization of memory and PS is especially popular among psychologists rather than among neurophysiologists. For it is precisely by neurophysiological facts that the theory of the informational function of PS is disproved. In this respect symptomatic are the positions taken by such well-known experts in sleep neurophysiology as Mancia [125] and Jouvet [92]. While considering sleep as an instinctive behavior, Mancia [125] formulates one of his principal points as follows: "The appetitive can be modified by learning, while the consummatory act is always "innate", in sleep it is easy to find a neurophysiological confirmation to that statement in the observation that REM phase is the only type of sleep which can be observed in intrauterine life and dominates the sleep pattern in newly born infants and animals. REM sleep progressively decreases as learning processes increase to give space to NREM sleep. Therefore, the hypothesis seems reasonable that REM sleep may be regarded as "innate" whereas NREM sleep increases in proportion to learning and sensorimotor development". In discussing this statement one may argue about the qualification of different types of sleep as different phases of instinctive behavior but not about the lack of correlation between the volume of informational work of the brain and the amount of PS.
Jouvet [92], considering the facts of the functional significance of PS, comes to the conclusion: "Finally, there has not yet been any unequivocal showing that the selective suppression of PS results in a specific disturbance of learning or of long-term memory since most of the experiments have used instrumental deprivation which induces many unspecific effects due to stress. In adult men, the long-term suppression of PS with MAO inhibitors or with chlorimipramine does not seem to interfere with any learning or memory processes".
In discussing the interrelations of memory and PS particular interest attaches to facts which deal with the dynamics of PS during a deficit of information arrival in the brain. Such a situation obtains: (a) in animals with section of the brain stem [81], (b) in animals with complete deafferentation sectioning of I, II, V, VIII, IX, X nerves and section of the dorsal spinal cord [219], in these conditions PS, unlike SWS does not appear to undergo any dramatic changes.
Experimental study of the interrelationship between memory and sleep both in man and in animals was undertaken in two directions; on the one hand, study was made of the effect of prelearning on the subsequent sleep-wakefulness cycle, and on the other, of sleep deprivation on learning. Data obtained on man appear to be more: non-uniform and contradictory than those obtained in animals. Thus, for example, Zimmerman et al. [227] described an increase in PS following tiresome learning with the use of distorting glasses. However, Allen et al. [5] have not confirmed these data. Later on there appeared papers supporting [27, 133] as well as rejecting [19, 83, 84, 144] the occurrence of PS increase in the post-learning period. Nevertheless, a number of authors point out either a preferential significance of slow wave sleep [48, 49, 61, 72, 110, 111, 126, 226] or a significance of a functional interrelation between SWS and PS [110, 111] in the organization of verbal learning. Bertini [19, 20], in summarizing the Basel symposium on "Information Processing and Learning during Sleep" at the First European Congress on Sleep Research, comes to the conclusion that there are no convincing data on the significance of PS for these processes.
In a critical appraisal of data on this topic, Webb [221], too, finds it impossible to come to a definite conclusion concerning the function of sleep in general and, in particular its role in the organization of memory. In all probability, the reason for this is that in the overwhelming majority of cases one or another phase of the sleep-wakefulness cycle was studied in isolation. Now, deprivation of one or another phase inevitably leads to the substitution of one physiological state of the brain for another, which can hardly be expected to result in any considerable disturbances in the brain integrative activity, in general, and a disturbance in the organization of memory, in particular. This accounts for the circumstance that even in the case of prolonged PSD under the influence of monoaminoxidase inhibitors no disturbances were noted in the mental sphere of human beings. In some cases in man [227], and in the majority of cases in animals [see 220 for ref.] learning and especially sleep deprivation were carried out in stressful situations which, causing as they do non-specific changes in the CNS, could substantially alter the picture.
Most of the animal studies on the interrelationship of memory and sleep deal with the role of PS in the consolidation of memory traces [57, 58, 169, 170], though there are some papers indicating its significance for the organization of other stages of memory, such as remembering [108, 109]. Papers providing the results of studies on the effect of learning on the subsequent sleep-wakefulness cycle in animals are scanty [34, 114, 123, 199], however, the data are more uniform in the sense that almost in all papers PS augmentation under the influence of learning is reported. Yet there are some papers [101, 146, 191] indicating the absence of such an effect. Since the effect was observed for 60-180 min immediately after the cessation of a learning session PS augmentation is considered to coincide with the consolidation phase of memory traces. However, as these experiments were mainly carried out on rats with acquisition of a defence reaction, it is difficult to rule out the effect of the preceding highly emotional tension on the subsequent sleep-wakefulness cycle. In this respect it is more advisable to perform animal experiments to study the effect of learning with food or drink reinforcement on the subsequent sleep-wakefulness cycle. In our experiments on cats [146, 162], in the course of acquisition of instrumental alimentary reflexes, no changes were observed in the PS amount after the learning sessions. A possible enhancement of the need for PS under the influence of the preceding learning session was examined by us by another, as it seems to us, more refined technique. In particular, we induced PSD by non-emotional awakening in both the control 5h sleep-wakefulness cycle and in cycles following the learning sessions. In these conditions the number of PS onsets per standard unit of time may point to the change of need for this phase under the influence of learning. And in the present case no evidence was obtained to indicate an increase of need for PS under the influence of learning. Moreover, in these experiments, under a systematic selective PSD in a 6 h sleep-wakefulness cycle, acquisition of instrumental alimentary reflexes to two feeders based on sound discrimination was not found to be delayed following each daily session (Fig. 1). Consequently, the lack of PS in that phase of memory organization in which the traces should have consolidated did not slacken the rate of learning. This fact indicates that replacement of PS by a physiological state in the sleep-wakefulness cycle not only fails to prevent but even has no retarding effect contrary to the statement, e.g. by Fishbein and Gutwein [58] on the memory trace consolidation. Further, our experiments have shown that acquisition of instrumental alimentary reflexes to two feeders with sound discrimination occurs in a normal way also when PSD is effected by the water tank procedure for 5 h after each daily learning session (Fig. 2). Recording of the electrical activity of the neo- and archipaleocortex as well as of the cardiac rhythm under conditions of the PSD water tank procedure reveals distinct changes in the animal's emotional sphere (elevation of the hippocampal theta rhythm, increased cardiac rhythm, etc.). However, in the subsequent period (from 17.00 p.m. to 10.00 a.m. of the following day), during which animals could sleep normally till the next learning session, these changes completely level off.
If PSD via the water tank procedure takes the whole intersession period, and such a situation lasts for a few days, the conditioned reflex activity then undergoes marked changes. At the beginning 48 h PSD does not affect the acquisition of instrumental alimentary reflexes and conditioned signal discrimination after the third learning session attains almost 100 % in both the experimental and control groups (Fig. 3). However, further continuous deprivation eventually impairs CS discrimination up to its complete disruption. This appears to be the effect of some nonspecific factor rather than the result of memory mechanism disturbance, for suffice it to allow the animals normal sleep for 24 h and CS discrimination recovers. Thus, even prolonged PSD via the water tank procedure fails to affect tangibly the memory mechanisms. The observed changes are, in all likelihood, due to changes in the emotional sphere and CNS excitability caused by the animal's long exposure to a stressful situation.
The studies of the effect of PSD by the water tank procedure on the acquisition of the active avoidance task in rats contain contradictory data: both impairment of learning [66, 170, 172, 202] and absence of the effect [4, 96, 193] as well as facilitation of learning [34, 104, 178, 207] are described. These studies involved mainly proactive PSD and a daily learning session (120 or 150 pairings of a conditioned signal with electrical stimulation) immediately after PSD via the water tank procedure. Our data, obtained in studies of the proactive effect of PSD, are in line with those of a majority of authors using the same procedure. In the deprived animals the learning criterion was reached at a smaller number of pairings of conditioned and unconditioned stimuli than was the case in the control ones (Fig. 6). This fact cannot, of course, be taken to indicate a negative effect of PS on learning. Open field behavior study of rats easily detects the changes in the animal's emotional sphere which result from PSD. Of these changes attenuation of fear (as evidenced by a decrease in defecation and urination) and enhancement of motor activity must be especially important. In accordance with these changes the deprived rats show a better space orientation, respond with more coordination to a conditioned signal, and are quicker learners of the avoidance reaction.
Of considerable interest for the evaluation of PS role in memory trace consolidation or conversion of short- into long-term memory are the results of those experiments in which rats were given one session active avoidance training following PSD and 3 days later, during which the rats were allowed to sleep normally, they were tested for retention. During that time PS presence in the sleep-wakefulness cycle of the rats apparently increased [37, 140] due to a rebound, and this increase coincided with the memory trace consolidation phase. If this question is considered from the position [see 58] that PS plays a major role in memory trace consolidation one should expect a better retention of a learned response in experimental rats as compared with controls. However, in the retention test the control animals did not differ from the experimental (Fig. 7) despite the fact that after pretraining PSD these latter learned avoidance faster than the former (Fig. 6). This finding indicates that PS augmentation following a learning session does not have any considerable effect on memory trace consolidation, thereby casting doubt on the significance of PS augmentation under the influence of pretraining learning [27, 114, 123, 199, 227]. Apparently, such PS augmentation is due to other factors (e.g. overstrain during hard learning) and is not directly related to memory trace consolidation processes.
In this aspect the most convincing facts were obtained in special experiments in which rats were given one-session active avoidance training following 48 h PSD and then were divided into two groups. The first group was allowed to sleep normally over the following 24 h and the second was subjected to further PSD for the same period. 24 h after the learning session all the animals were tested for retention of the learned response. In this case the active avoidance acquisition was conducted under similar conditions for all rats, but subsequently the situation was changed. Group I animals that slept normally during the intersession period displayed an obvious increase of PS presence, whereas in Group II animals PS was completely absent during that period. If it is borne in mind that the intersession time must embrace a considerable part of the consolidation phase, the significance of the data obtained in the present experiment for assessing the role of paradoxical sleep in the conversion of short- into long-term memory will become clear. Neither an increased presence of PS nor its absence in the consolidation phase was found to affect the memory of an active avoidance response acquired during one-session learning. The fact that in the testing session deprived rats reached the learning criterion quicker than those having a rest during the intersession period can be explained by the changes in the animals' emotional sphere which, in turn, are due to a stressful situation attending the water tank procedure [148]. This is supported by data on open field behavior in rats undergoing 24, 48 and 72 h PSD by the water tank procedure (Fig. 22 - 24). Deprived rats exhibited increased motor activity in parallel with decreased fear reaction. Such changes in the emotional sphere create a proper background for successful active avoidance learning.
The experiments of Kovalzon and Tsibulsky [106] strongly support the suggestion that changes in the emotional sphere resulting from PSD water tank procedure are due to a lack of the given phase of the stressful nature of this procedure. In their experiments PSD was effected by electrical stimulation of the mesencephalic reticular formation with such parameters which caused only non-emotional awakening of the animal. Such manipulations - conducted even for several days - were not found to cause stressful changes revealed by the open field method. No changes in the emotional sphere were observed in cats if PSD was given in the usual experimental chamber by the method of non-emotional awakening. These findings are not in line with the viewpoint of Fishbein and Gutwein [58] that changes produced by the PSD water tank procedure should be considered as resulting from the absence of the given phase of the sleep-wakefulness cycle rather than stemming from the stressful situation intrinsic to this procedure.
If some factor affects the consolidation of memory traces, the effect must be most pronounced in its retroactive rather than proactive influence [see 7, 82, 129, 130, 137]. From this it is natural to assume that PSD, too, may affect learning most effectively when it is effected in the consolidation phase and not prior to it. However, our data obtained in studies of the post-trial effect of PSD on the acquisition of active avoidance reaction fail to reveal any specific significance of PS in learning. It was ascertained that PSD, via the water tank procedure effected in the intersession period of active avoidance task learning, (a) does not retard the acquisition of an alarm reaction (Fig. 11) and (b) tangibly facilitates the acquisition of first irregular reactions of active avoidance (Fig. 12).
The deprived rats reach the criterion of optimal acquisition of avoidance reaction more rapidly (9 correct responses out of 10 trials) than do the control ones (Fig. 13). However, if PSD via the water tank procedure continues more than 72 h, then it starts to have a negative effect on the acquired habits, which is, in all probability, the result of an accumulation of nonspecific changes in the emotional sphere and CNS excitability produced by the stressful situation during deprivation.
Post-trial deprivation of PS does not retard the consolidation of memory traces even when it follows the termination of a one-day learning session. To elucidate this, two groups of rats were compared in our experiments. In the control group, in one-day session the number of trials after which the animals reached the learning criterion was determined (9 correct responses out of 10 trials). 48 h later they were tested for the retention of the acquired reactions. In the exper imental group the one-day session was followed by 48 h PSD in a water tank. In the experimental group the retention of the acquired habit proved to be no worse than it was in the control group. Moreover, in this respect, rats of the experimental group seemed to perform much better, which is again explained by the attenuation of fear and enhancement of motor activity resulting from PSD.
Thus, the above-described findings by no means indicate that, in contrast to other phases of sleep, PS has some specific relevance to learning and memory.
Most extensive experiments were carried out to study the effect of PSD on learning in a passive avoidance test [see 57, 58, 220]. This may apparently be explained by a number of circumstances. Firstly, a simple methodological procedure allows simultaneous experimentation with a considerable number of animals, yielding an adequate body of data for statistical treatment. Secondly, during passive avoidance learning one may readily differentiate from one another different steps of memory organization and to induce at will either a proactive or retroactive effect of PSD. This is especially important in studying this effect on the consolidation of memory traces.
It is not accidental that in analysing the results of a study of the effect of PSD on learning in a passive avoidance test almost all investigators note a disturbance or retardation of the development of the memory trace consolidation phase [55, 56, 178, 190]. And thirdly, passive avoidance task learning reveals with particular clarity the significance of those nonspecific changes which necessarily attend PSD via the water tank procedure. It is the evaluation of the significance of these nonspecific changes that is the source of all differences in views concerning the role of PS in memory organization. As indicated above, these differences are most fully reflected in the review articles by Vogel [220], Fishbein and Gutwein [58].
Analysing at length the data reported by the proponents of the consolidation theory of PS, Vogel [220] substantiates the view that memory deficit resulting from PSD is actually due to changes in the emotional sphere which, in turn, are produced not by a lack of PS but by a stressful situation attending the use of the water tank procedure. At the same time, he makes reference to the papers in which a negative effect of stress on learning in a passive avoidance test is shown [22, 63, 181]. In Vogel's [220] opinion these changes predominantly affect not the mechanism of retention or consolidation of memory traces, but that of retrieval. In spite of this, in his review the nature of memory deficit under these nonspecific changes in emotionality remains an open and debatable question. It was precise by this point that served as a bench mark for Fishbein and Gutwein [58] in their second review of the data on the role of PS in the processes of learning and memory. This is what they say on this matter in connection with Vogel's position: "In a recent review of the PSD literature Vogel [220] has presented a view that is both consistent with our own position and contradictory. His position, and ours, is that PSD heightens central neural excitability and affects motivational behavior by producing alterations in neurochemical and neurophysiological mechanisms. We differ from Vogel in that we believe these alterations to be part of the mechanisms that underlie memory storage processes. Vogel, on the other hand, implies that mechanisms subserving memory processing are separate and distinct from PSD-induced alterations in neuronal excitability and changes in motivational behavior. We believe that they are inseparable.
In our view our analysis casts doubt on the conclusion drawn by Vogel, that "the weight of evidence indicates that ... the effects of REM sleep deprivation on retention and on new learning remain unclear". In sum, experiments which have eliminated sources of experimental variability all report consistent results: "PSD via the water tank procedure is selective and can result in alterations in neuronal excitability, changes in motivational behavior, and amnesia of learned behaviors" [58, p. 433].
However, this is not precisely the case. Vogel [220] indicates clearly that memory retention, storage of memory traces and conversion of short-term memory into long-term under PSD should not vary. In his view, changes produced by a stressful situation under PSD via the water tank procedure must affect the retrieval of memory traces rather than their consolidation, as stated by Fishbein and co-workers [55-59, 121]. These are certainly two different positions of interpretation of the foregoing data. At present, in view of the accumulation of new evidence the picture becomes clearer. Thus, for example, in the opinion of Fishbein [55], Segales, Domino [190], Linden et al. [121] PSD prior to the learning of a passive avoidance task has no effect on the acquisition of the task and on long-term memory, whereas conversion into long-term memory is disturbed. Miller et al. [134], as well as Kruglikov et al. [108, 109] have not confirmed this. Our experiments show that a preliminary 72 h PSD has no marked effect on the long-term retention of the learned skill (habit) task. If in 72 h PSD deprived rats via the water tank procedure electrical shock is applied in the experimental chamber for testing passive avoidance, they, like the control ones, avoid this situation not only after a few hours but also 24 and 48 h within the application of shock (Fig. 21). This fact is quite understandable from the position of the significance of nonspecific emotional changes produced by a stressful situation resulting from PSD the water tank procedure. Our experiments in which we studied the behavior of rats deprived in open field show that in 24-48 h of a normal sleep-wakefulness cycle these changes completely level off (Fig. 23); naturally they cannot act as an interfering factor when the retention of the acquired skill (habit) task is examined. Thus, it may be concluded that a preliminary PSD, even via the water tank procedure, has no effect on the conversion of short-term memory into long-term. Understandably, the post-trial action of PSD on learning and memory proves to be more effective in the passive avoidance test. Relevant data in the literature are more uniform [see 58]. Thus, the majority of authors indicate that PSD via the water tank procedure following learning in a passive avoidance test disturbs memory. However, it is essential to note that in all of these papers memory is considered to be disturbed if the deprived rats, in contrast to the controls, do not reach 5 or 10 min criterion, i.e. if it takes them 5 or 10 min to penetrate from the light (safe) compartment into the dark (dangerous) compartment of the experimental chamber. Unfortunately, almost no or insufficient attention has been paid to the way the latency alters during penetration into the dangerous compartment in the deprived rats, as compared with the latency before the application of electrical shock.
In analysing our experimental data the following points attract attention: 1. Part of the animals in which the application of electrical shock was followed by 24 or 48 h PSD do not reach the 5 min criterion of staying in the safe compartment of the experimental chamber for studying the memory of passive avoidance task. However, the percentage of such animals is smaller following 48 h rather than 24 h PSD (Fig. 15). Characteristically enough, not all control rats reach this criterion. 2. In all animals which, following both 24 h and 48 h PSD, fail to reach the above criterion there is a sharp increase in the latency of penetrating the dangerous compartment from the safe one, as compared with the latency before the application of an electrical shock (Fig. 16). Moreover, this increase was more pronounced following 24 h rather than 48 h PSD (Fig. 16A) than following 48 h (Fig. 16B), though in the latter case, too, it is statistically highly significant. The only conclusion one can come to in analysing these data is that the increase in the latency of penetration into the dangerous compartment in both deprived and control rats is due to the retention in memory of the electrical shock received in the dangerous compartment. 3. This conclusion is confirmed by visual observations of the rats' behavior: they show signs of fear when they are in the safe compartment, and especially, when entering the dangerous part of the chamber. 4. Rats (both deprived and control), reaching the 5 min criterion of staying in the safe compartment, in the experimental chamber show higher motor and exploratory activity, compared with the rats which reach this criterion. It appears that PSD results in the enhancement of motor activity, attaining an optimal level following 48 h deprivation (Fig. 23), though it is markedly pronounced after 24 h deprivation too. Data on open field behavior studies reveal that, apart from the enhancement of motor and exploratory activity, PSD via the water tank procedure causes attenuation of the fear reaction. All this might cause the deprived rats to be bolder in entering the dangerous part of the chamber in spite of retention in memory of the electrical shock received there. A clear competition is observable here between the reaction of fear, which for some reason is attenuated as a result of PSD via the water tank procedure, and the exploratory activity, which is strongly increased in deprived rats. This competition results in the final resolution to penetrate into the dangerous compartment rather than in a disturbance of memory mechanisms in general, and conversion of short-term memory into long-term, in particular, or a disturbance of the stability of the latter [see 58]. From these facts it is also evident that PSD does not disturb the retrieval of memory traces either.
If the increase of CNS excitability [31, 32, 167] may be assumed to be the neurophysiological basis of enhancement of the motor and exploratory activity under PSD via the water tank procedure, then the neurophysiological basis for attenuation of the fear reaction remains unclear. It may be only conjectured that under such experimental conditions attenuation of fear seems to be rather an accessory phenomenon related to the transfer of the animal from a highly stressful situation, in which the fear reaction is on a high level, to a less stressful one, in which this reaction attenuates drastically and the animal is rendered bolder than usual. It may be also supposed that in a highly stressful situation a fear reaction becomes habituated, as it were, the threshold for its evocation increases and, after the animal's transfer to a less stressful situation, the environmental elements appear to be ineffective in the sense of evocation of a fear reaction.
To elucidate the question as to what accounts for the above described shifts in the emotional sphere, i.e. a stressful situation attending the use of the water tank procedure or PSD proper, special significance attaches to our data obtained on cats. As indicated above, deprivation of PS in cats through non-emotional awakening did not entail the changes (enhancement of brain excitability, hyperphagia, aggressiveness, etc.) that are observed during PSD via the water tank procedure. It may be conjectured that at the use of PSD, not resulting in a stressful situation, no changes will be observed in the emotional sphere. In this connection, reference should be made to the papers of Kovalzon and Tsibulsky [106]. In their experiments PSD via electrical stimulation of the RF did not cause alterations in the rat's emotional behavior.
In elucidating the role of emotional shifts produced by PSD via the water tank procedure in the alteration of the rats' behavior in the experimental chamber as tested for passive avoidance our results obtained from studies of pairing of PSD with rest are worth noting. As described above, rats which, following electrical shock, had been subjected to 24 h (Fig. 17) or 48 h (Fig. 18) PSD and in the subsequent 24 h period had been allowed a normal course of the sleep-wakefulness cycle, when tested for the retention of the habit did not differ from the controls, whereas at reverse pairing of rest and PSD, they behaved in the same way as the animals deprived without a preliminary rest. Thus, neither 24 h nor 48 h PSD has a marked effect on the rats' behavior in a passive avoidance test, if after deprivation time is afforded for the leveling off of the changes in emotional sphere which result from a stressful situation. And conversely, if such shifts in the emotional sphere are produced by PSD via the water tank procedure given after enough time for the memory traces to be consolidated (24 and 48 h), they behave in the same way as the deprived rats without a preliminary rest. This implies that the emotional shifts produced by PSD via the water tank procedure do not disturb the consolidation of memory traces, but do alter the rats' behavior after the memory traces have been consolidated. This inference is supported by data obtained from studies of the effect of 48 h and 72 h PSD paired with a 5-6 h rest on the rats' behavior in the chamber for checking passive avoidance. Following PSD, a 5-6 h, rest with the maintenance of quite wakefulness was enough for the experimental rats to behave in the same way as the control ones (Fig. 20). In this case, too, recovery of behavior is related to the passing of the changes in the emotional sphere (Fig. 24). This fact directly indicates, first, that the changes in the emotional sphere are related to a stressful situation in the deprivation chamber and not to PSD per se (since a strong demand for PS is fully maintained even after a 5-6 h rest) and, second, that neither PSD nor the changes produced by it in the emotional sphere retard the consolidation of memory traces, conversion of short-term memory into long-term, or stabilization of long-term memory traces.
In evaluating the changes in the emotional sphere produced by PSD via the water tank procedure it is particularly noteworthy that under these conditions learning of active avoidance is facilitated and retrieval of the learned passive avoidance task seems to be hindered. If PS played a specific role in the organization of learning and memory, then its deprivation would equally hinder the acquisition of a conditioned habit of both active and passive avoidance. The variously directed effect of one and the same change in the emotional sphere on the learning of the two different tests is rendered quite understandable if one bears in mind that enhancement of motor- and exploratory activity arid attenuation of the fear reaction provide a good background, on the one hand, for the acquisition of active avoidance and on the other, for the shortening of the latency of penetrating into the dangerous compartment of the experimental chamber from the safe, one in testing for passive avoidance. In this connection it is interesting to note that Levin and Brush [118] and Levin [117] have demonstrated that stress may facilitate active avoidance learning.
The absence of PSD effect on learning and memory does not at all imply that this state of the brain has no significance for the organization of mental processes in general, and learning in particular. However, the method of deprivation, changing one physiological state in the sleep-wakefulness cycle to another, apparently fails to reveal the part played by one or another phase in mental processes. There appears to be no qualitative difference between wakefulness and PS. Even those structures of the brainstem RF which were earlier regarded as triggering PS [13, 25, 26, 68, 75-77, 127] are activated in PS and in active wakefulness on an equally high level [23, 29, 132, 194-197]. When PS is deprived, the latter is mainly replaced by wakefulness [163, 164]. In this respect the slow wave phases may hold a special place, but it is so far difficult to speak about the qualitative differences delimiting this state from others. One may definitely speak only of the attenuation of the interference of information received by the brain in SWS - as compared with wakefulness and PS - with some other information that may arrive in the brain after it. Neurophysiological findings show that the activity of the brain in SWS fluctuates less than it does in wakefulness and PS [160]. This especially concerns the structures involved in the system of wakefulness which must be the main links of regulation of informational processes, for it is precisely during wakefulness that the bulk of information (especially on the events of the external world) arrives. Attenuation of interfering influences should be particularly important in the phase of memory trace consolidation. For it is just this phase that is most susceptible and vulnerable of all the phases in memory organization [82, 129, 130, 137, 157, 158]. If the realization of one or another step of memory organization re quires a high level of brain activity, and particularly of the structures of wakefulness, then such physiological states as wakefulness and PS do not differ in this respect from each other. This is evidenced best by the results obtained in studies of the dynamics of neuronal activity from the mesodiencephalic structures of the wakefulness system in the sleep-wakefulness cycle [153, 155, 157-160]. They indicate that neuronal activity of these structures is at its highest during active emotional wakefulness and in the emotional stage of PS, their neuronal activity substantially attenuating in SWS. It is important to note that neuronal activity of the mesodiencephalic activating structures (i.e. structures involved in the system of wakefulness) during wakefulness and PS fluctuates equally within a wide range, while in SWS the degree of this fluctuation diminishes dramatically. In SWS, neuronal activity of the sleep system is augmented, compared with wakefulness and PS, but the augmentation proceeds monotonously, so that, figuratively speaking, this physiological state differs from other states by the diminution of the "noisiness" of the brain.
The position that consolidation of memory traces and conversion of short-term memory into long-term is the function of PS is often argued by reference to neurochemical findings as well [58], according to which it is precisely in PS [44, 45] that the protein content in a perfusate from the brainstem reticular formation attains its peak [44, 45]. However, in these studies other states are usually compared with quiet non-emotional rather than with active emotional wakefulness, PS being the letter's analogue in terms of the level of brain activity. There is evidence that PSD affects the incorporation of amino acids into the brain proteins [see 58], but this can be readily related to those non-specific changes in the emotional sphere that attend PSD via the water tank procedure. That the inhibition of protein synthesis is paralleled by a disturbance of conversion of short-term memory into long-term and suppression of PS cannot serve as a convincing argument in favour of the informational function of the latter [30, 173, 200, 203]. In such experiments it is important to study in the first place, how the sleep-wakefulness cycle is altered as a whole. If there is an increase in the amount of wakefulness this is quite enough for the suppression of PS. As to the disturbance of consolidation of memory traces and conversion of short-term memory into long-term, for this to occur inhibition of protein synthesis in wakefulness is no less important than it is in PS. Yet, the major fact against the above arguments is that PSD does not result in the disturbance of consolidation of memory traces underlying the conversion of short-term memory into long-term. This is clearly indicated by the facts described in the present paper.
In this respect, reference to the role of brain transmitters in the organization of memory and to their dynamics in different physiological states [see 58] seems unconvincing. Both acetylcholinergic [99, 100] and catecholaminergic synapses [15, 107, 115] may play an important role in the organization of memory (otherwise it is hard to conceive the work of the brain required for this function). However, beaming in mind the dynamics of the number of relevant transmitters in the sleep-wakefulness cycle, it is barely reasonable to speak of the preferential role of PS in this aspect. After all, these systems operate intensively not only during PS but during wakefulness as well [21, 62, 85, 86, 120]. At the same time if one bears in mind that the state of wakefulness holds a more prominent place in the cycle both in volume and in significance, then the illogicality of the above reasoning will become clearer. In this relation paramount importance attaches to the fact that the brain system of wakefulness shows equally high activity both in wakefulness and in PS. Even such a specific structure as the locus coeruleus, involved in the regulation of PS, judging from its neuronal activity [29], functions equally efficiently during wakefulness and PS. Does not this speak in favour of the identity of the work of the relevant transmitter (noradrenaline) system in the phases of wakefulness and PS?
In conclusion, it may be observed that so far there is no convincing evidence on the specific role of PS in the regulation of learning and memory. The results obtained by the method of PSD unfortunately give a negative answer to this question. The data presented here, however, do not provide a conclusive answer either. PS is so rich in both physiological and psychical content that it must certainly participate in the regulation of learning and memory. But what is its specific role? Does its function differ in this regard from that of other phases, and especially from the function of wakefulness? These points, unfortunately, still remain obscure. Perhaps, in this respect, too, the sleep-wakefulness cycle should be considered as a functional entity in which each has its place, all of them being intimately interrelated, to study the specific role of one of them is extremely difficult. Differentiation of the functions of wakefulness and PS in terms of their participation in the consolidation of memory traces and conversion of short-term memory into long-term remains the most difficult problem.
Recently authentic experimental data have been reported on the role of PS in the long-term storage of memory traces by way of retrieval of past experience in the form of previously elaborated conditioned reflexes (see in the present volume p. 214).
l. Abuladze, G.V., Loskutova, L.V., Ilyuchonok, R.Yu. Effect of polarization of amygdaloid complex on elaboration of conditioned defence reaction in rat. Zh. Vyssh. Nervn. Deyat., 1976, 26, 2: 418-420, (in Russian).
2. Adams, P.M. and Barrat, E.S. Nocturnal sleep in squirrel monkeys. Electroenceph. Clin. Neurophysiol., 1974, 36: 201-204.
3. Adey, W.R. EEG studies of hippocampal system in the learning process. In: Physiologic de l'hippocampe. Paris, Centre nationale de Recherche Scien-tifique, 1962: 203-222.
4. Albert, J., Cicala, G.A., Siegle, J. The behavioral effects of REM sleep deprivation in rats. Psychophysiol., 1970, 6, 5: 550-560.
5. Allen, S., Oswald, J., Lewis, S., Tagney, J. The effect of distorted visual input on sleep. Psychophysiol., 1972, 9: 111.
6. Allison, T., Van Twyver, H., Goff, W.R. Electrophysiological studies on the echidna Tachyglossus aculetus. 1. Waking and sleep. Arch. Iial. Bio., 1972, 110: 145-184.
7. Alpern, H.P. and McGaugh, J.L. Retrograde amnesia as a function of duration of electroshock stimulation. J. Comp. Physiol. Psychol., 1968, 65: 265-269.
8. Aserinsky, E. and Kleitman, N. Regularly occurring period of eye motility and concomitant phenomena during sleep. Science, 1953, 118: 273-274.
9. Aserinsky, E. and Kleitman, N. Two types of ocular motility occur ring in sleep. J. Appl. Physiol., 1955, 8, 1: 11-18.
10. Astic, L. and Royet, J. P. Someil chez le rat - kangourou (potarul apicalos). Etude chez l'adulte et chez le jeune un moile avant la sortie definitive du marsupian effects du sevrage. Electroenceph. Clin. Neurophysiol., 1974, 37: 483-489.
11. Baldwin, B.A. and Soltysik, S.S. Asquisition of classical conditioned defence responses in goats by cerebral ishemia. Nature, 1965, 206: 1011-1013.
12. Baldwin, B.A. and Soltysik, S.S. Studies on the nature of recent memory. Acta Biol. Exp., 1969, 29: 293-318.
13. Batini, C., Magni, F., Palestini, M., Rossi, G., Zanchetti, A. Neural mechanisms underlying the enduring EEG and behavior activation in the midpontine pretrigeminal cat. Arch. Ital. Biol., 1959, 97: 13-25.
14. Bekhterev, V. Demonstration eines Gehirns mit Zerstorung der vorderer und innerer Theile der Hirnrinde beider Schlafenlappen. Neurol. Zbl., 1900, 19: 990-991.
15. Benett, E.L., Diamond, M.C., Krech, D. Chemical and anatomical plasticity of the brain. Science, 1964, 146: 610-619.
16. Beritashvili, I.S. Phylogeny of memory development in vertebrates. In: A.G. Karczmar and J.C. Eccles (Eds) Brain and Human Behavior. New-York, Springer Verlag, 1972.
17. Bert, J. Similitudes et differences du sommeil chez deux babouins, Papio hamadryas et Papio papio. Electroenceph. Clin. Neurophysiol., 1973, 35: 209-212.
18. Bert, J., Kripke, D.F., Phopes, J.M. Electroencephalogram of the nature chimpanzee: 24-hour recordings. Electroenceph. Clin. Neurophysiol., 1970, 28: 368-373.
19. Bertini, M. REM sleep as a psychophysiological "egency" of memory organization. In: Sleep 1972: Physiol., Biochem., Psychol., Pharmacol., Clinical implications. 1st Europ. Congr. Sleep Res., Basel, Karger, 1973: 61-62.
20. Bertini, M. and Torre, A. REM sleep and memory consolidation in humans. In: Sleep 1972: Physiology, Biochemistry, Psychology, Pharmacology, Clinical implications. 1st Europ. Congr. Sleep Res., Basel, Karger, 1973: 453-457.
21. Bolme, P., Fuxe, K., Lindbrink, P. On the function, of central catecholamine neurons. Their role in cardiovascular and arousal mechanisms. Res. Communs. Chem. Pathol. Pharmac., 1972, 4: 657-697.
22. Booth, D. Neurochemical changes correlated with learning and memory. In: G. Unger (Ed) Molecular Mechanisms in Memory. New York, Plenum. Press, 1970: 1-58.
23. Bour, H.L. and Corner, M.A. Bioelectric activity of pontine reticular neurons during the natural sleep-waking cycle and after clomipramine administration in young rats. Neurosci. Lett., 1981, 24, Suppl. 7: 485.
24. Bureš, I. and Burešova, O. Cortical spreading depression as a memory disrupting facter. J. Comp. Physiol. Psychol., 1963, 56: 268-271.
25. Candia, O., Rossi, G., Sekino, T. Brain stem structures responsible for the electroencephalographic patterns of desynchronized sleep. Science, 1967, 155: 720-722.
26. Carli, G. and Zanchetti, A. A study of pontine lesions suppressing deep sleep in the cat. Arch. Ital. Biol., 1965, 103: 751-788.
27. Cartwright, R.D. Problem solving in REM, NREM and waking. Psychophysiol., 1972, 9: 108.
28. Chorover, S.J. and Schiller, P.H. Short-term retrograde amnesia in rats. J. Comp. Physiol. Psychol., 1965, 59: 73-78.
29. Chu, N.S. and Bloom, F.E. Norepinephrine containing neurons: changes in spontaneous discharge patterns during sleeping and waking. Science, 1972, 179: 908-910.
30. Cohen, H.B. and Barondes, S.H. Effects of acetoxycycloheximide on learning and memory of a light-dark discrimination. Nature, 1968, 218: 271-173.
31. Cohen, H.B. and Dement, W.C. Sleep: changes in threshold to electroconvulsive shock in rats after deprivation of "paradoxical" phase. Science, 1965, 150: 1318-1319.
32. Cohen, H.B., Thomas, J., Dement, W.C. Sleep styles, REM deprivation and electroconvulsive threshold in the cat. Brain Res., 1970, 19: 317-321.
33. Coons, E.E. and Miller, N.E. Conflict versus consolidation of memory traces to explain "retrograde amnesia" produced by ECS. J. Comp. Physiol. Psychol., I960, 53: 521-531.
34. Delacour, J. and Brenot, J. Sleep patterns and avoidance conditioning in the rat. Physiol. Behav., 1975, 14: 329-335.
35. Dellaire, A. and Brickenbush, Y. Rest-activity cycle and sleep patterns in captive foxes (Vulpes Vulpes). Experientia, 1974, 30: 59-60.
36. Dement, W. The occurrence of low voltage fast electroencephalogram: patterns during behavioral sleep in the cat. Electroenceph. Clin. Neurophysiol., 1958: 10: 291-296.
37. Dement, W.C. The effect of dream deprivation. Science, 1960, 131: 1705-1707.
38. Dement, W.C., Henry, P., Cohen, H., Fergusson, G. Studies on the effect of REM deprivation in humans and in animals. Res. Publ. Ass. Nerv. Ment. Diss., 1967, 45: 456-468.
39. Dement, W. and Kleitman, N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility and dreaming. Electroenceph. Clin. Neurophysiol., 1957, 9: 673-690.
40. Dement, W. and Kleitman, N. The relation of eye movements during sleep to dream activity. An objective method for the study of dreaming. J. Exp. Psychol., 1957, 53: 339-346.
41. Deneberg, V.H. Open-field behavior in the rat: What does it mean? Ann. N. Y. Acad. Sci., 1970, 159: 852-859.
42. Dewan, E.M. The programming (P) hypothesis for REM sleep. In: E. Hartmann (Ed) Sleep and Dreaming. Little, Brown. Boston, 1970: 295-307.
43. Douglas, R.J. The hippocampus and behavior. Psychol. Bull., 1967, 67(6): 416-442.
44. Drucker-Colin, R.R. and Spanis, C.W. Is there a sleep transmitter? Prog. Neurobiol., 1976, 6: 1-22.
45. Drucker-Colin, R.R., Spanis, C.W., Cotman, C.W, McGaugh, J.L. Changes in protein levels in perfusates of freely moving cats: Relation to behavioral state. Science, 1975, 187: 963-965.
46. Duncan, C.P. Habit reversal induced by electroshock in the rat. J. Comp. Physiol. Psychol., 1948, 41: 11-16.
47. Duncan, C.P. The retroactive effect of electroshock in learning. J. Comp. Physiol. Psychol., 1949, 42: 32-34.
48. Ekstrand, B.R. Effects of sleep on memory. J. Exp. Psychol., 1967, 75: 64-73.
49. Ekstrand, B.R., Barrett, T.R., West, G.N., Maier, W.G. The effect of sleep on human long-term memory. In: R.R. Drucker-Colin and J.L. McGaugh (Eds) Neurobiology of Sleep and Memory. Academic Press, New York, 1977: 419-439.
50. Elazar, Z. and Adey, W. Electroencephalographic correlation of learning in subcortical and cortical structures. Electroenceph. Clin. Neurophysiol., 1967, 23: 306-319.
5l. Ephron, H.S. and Carrington, P. Rapid eye movement sleep and cortical homeostasis. Psychol. Rev., 1966, 73: 500-526.
52. Evarts, E.V. Activity of neurons in visual cortex units during sleep with fast EEG activity. J. Neurophysiol., 1962, 25: 812-816.
53. Evarts, E.V. Temporal patterns of discharge of pyramidal tract neurons during sleep and waking with monkey. J. Neurophysiol., 1964, 27: 152-171.
54. Fergusson, J. and Dement, W.C. The behavioral effect of amphetamine on REM deprivated rats. J. Psychiatr. Res., 1969, 7: 11-118.
55. Fishbein, W. Interference with conversion of memory from short-term to long-term storage by partial sleep deprivation. Commun. Behav. Biol., 1970, 5: 171-175.
56. Fishbein, W. Disruptive effects of rapid eye movement sleep deprivation on long-term memory. Physiol. Behav., 1971, 6: 279-382.
57. Fishbein, W. Alterations of the sleep states: Sleep deprivation: total, partial and selective. In: M.H. Chase (Ed) The Sleeping Brain. Los Angeles: UCLA Brain Information Service, 1972: 347-353.
58. Fishbein, W. and Gutwein, M. Paradoxical sleep and memory storage processes. Behav. Biol., 1977, 19: 425-464.
59. Fishbein, W., Kastaniotis, C., Chatlman, D. PS: prolonged augmentation following learning. Brain Res., 1974, 79: 61-75.
60. Fisher, C. Psychoanalytic implications of recent research on sleep and dreaming. I. Empirical findings. J. Amer. Psychoanal. Ass., 1965, 13: 197-270.
61. Fower, M.J., Sullivan, M.J., Ekstrand, B.R. Sleep and memory. Science, 1973, 179: 70-72.
62. Fuxe, K. and Lidbrink, P. Biogenic amine aspects of sleep and waking. Sleep, 1972: Physiology, Biochemistry, Psychology, Pharmacology, Clinical implications. 1 st Europ. Congr. Sleep Res., Basel, Karger, 1973: 12-26.
63. Glassman, E. The biochemistry of learning: An evaluation of the role of RNA and protein. Ann. Rev. Biochem., 1969, 38: 605-616.
64. Gogolak, G., Stumpf, C., Petsche, H., Sterc, J. The firing patterns of the septal neurons and the form of the hippocampal theta wave. Brain Res., 1968, 7: 201.
65. Gray, Y.A., Feldon, Y., Rawlins, J. N. P., Owen, S., McNaughton, N. The role of the septo-hippocampal system and its noradrenergic efferents in behavioral responses to non-rewarding. In: Functions of the Septo-Hippocampal System. Ciba foundation Symposium 58, Elsevier, Oxford, New York, 1978: 275-307.
66. Hartman, E. and Stern, W. Desynchronized sleep deprivation: learning deficit and its reversal by increased catecholamines. Physiol. Behav., 1972, 8: 585-587.
67. Hebb, D.O. The organization of Behavior: a Neuropsychological Theory. J. Wiley and Sons, New York, 1949.
68. Henley, K. and Morrison, A.R. A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of PS in the cat. Acta Neurobiol. Exp., 1974, 34: 215-232.
69. Hennevin, E., Leconte, P., Bloch, V. PS increase triggered by learning, extinction and relearning of a response besed on positive reinforcement. Brain Res., 1974, 70: 43-54.
70. Hicks, R.A., Gomez, S., Gonzales, L., Kuroda, M., Orma, N.J., Reyes, Y. REM sleep deprivation reduces emotionality in female rats. Bull. Psychonom. Sci., 1981, 17: 244-245.
71. Hicks, R.A. and Hirshfild, C., Humphray, V., Lamber, A., Giampaoli, S., Hawkins, S. REM sleep deprivation and food competition in male rats. Physiol. Behav., 1981, 26: 245.
72. Hicks, R.A., Moore, J.D. REM sleep deprivation diminishes fear in rats. Physiol. Behav., 1979, 22(4): 689-692.
73. Hishikawa, Y., Cramer, H., Kuhlo, W. Natural and melatonin induced sleep in young chickens. A behavioral and electrographic study. Exp. Brain Res., 1969, 7: 84-94.
74. Hobson, Y.A. Electrographic correlates of behavior in the frog with special reference to sleep. Electroenceph. Clin. Neurophysiol., 1967, 22: 113-121.
75. Hobson, J.A., McCarley, R.W., Pivik, T., Freedman, R. Selective firing by cat pontine brain stem neurons in desynchronized sleep. J. Neurophysiol., 1974, 37: 497-511.
76. Hoshino, K. and Pompeiano, O. Selective discharge of pontine neurons during the postural atonia produced by anticholinesterase in the decerebrate cat. Arch. Ital. Biol., 1976, 114: 244-277.
77. Hoshino, K., Pompeiano, O., Magherini, P.C, Margner, T. The oxillatory system responsible for the oculomotor activity during the burst of REM. Arch. Ital. Biol., 1976, 144: 278-309.
78. Huttenlocher, P.R. Evoked and spontaneous activity in single units of median brain stem during natural sleep and waking. J. Neurophysiol., 1961, 24: 451-468.
79. Ioseliani, T.K. and Chokheli, K. Effect of sleep on retention of memory of the acceptive objects in cats. In: Sovremenny Problemy Deyatelnosti i Stroenie Centralnoi Nervnoi Sistemy. Tbilisi, Metsniereba, 1976: 59-68 (in Russian).
80. Isaacson, R.L. The Limbic System. New York, London, Plenum Press, 1974.
81. Jenkins, Y.G. and Dallenbach, K.M. Oblivion during sleeping and waking. Am. J. Psychol., 1924, 35: 605-612.
82. John, E.R. Mechanisms of Memory. Acad. Press, New York, 1967.
83. Johnson, L.C. Are stages of sleep related to waking behavior? Amer. Sci., 1973, 61(3): 326-338.
84. Johnson, L.C. The effect of total, partial and stage sleep deprivation on EEC patterns and performances. In: Behavior Brain Electrical Activity. Plenum Press, New York, 1975: 1-30.
85. Jones, B.E. The respective involvement of noradrenaline and its deaminated metabolites in waking and paradoxical sleep: A neuropharmaeological model. Brain Res., 1972, 39: 121-136.
86. Jones, B., Bobillier, P., Pin, C., Jouvet, M. The effect of lesions of catecholamine-containing neurons upon monoamine content of the brain and behavioral waking in the cat. Brain Res., 1973, 58: 157-177.
87. Jouvet, M. Telencephalic and rhombencephalic sleep in the cat. In: G.E. Wolstenholme, O'Connor M. Churchill (Eds) The Nature of Sleep. London, 1961: 188-208.
88. Jouvet, M. Recherches sur les structures nerveuses et les meanisms responsable des differentes phases du sommeil physiologique. Arch. Ital. Biol., 1962, 100: 125-206.
89. Jouvet, M. The rhombencephalic phase of sleep. Progr. Brain. Res., 1963, I: 406-424.
90. Jouvet, M. Behavioral and EEG effects of paradoxical sleep deprivation in the cat. Proc. Int. Congr. Physiol. Sci., 1965, 87: 344-353.
91. Jouvet, M. The neurophysiology of the states of sleep. Physiol. Rev., 1967, 47: 117-178.
92. Jouvet, M. The role of monoaminergic neurons in the regulation and function of sleep. In: O. Peter-Quadens and J.D. Schlag (Eds) Basic Sleep Mechanisms. Academic Press, New York, London, 1974: 207-236.
93. Jouvet, M. and Delorme, F. Locus coeruleus et sommeil paradoxical. C. R. Soc. Biol. (Paris), 1965, 159: 895-899.
94. Jouvet, M., Michel, F., Courjon, J. L'activite electrique dur hinecephale au cours du sommeil chez le chat. C. R. Socr Biol., 1959, 153(I): 101-105.
95. Jouvet, D., Vimont, P., Delorme, F., Jouvet, M. Etude de la privation selective de la phase paradoxale de sommeil chez le chat. C. R. Soc: Biol. (Paris), 1964, 158: 756-759.
96. Joy, R.M. and Prinz, P.N. The effect of sleep altering environments upon the acquisition and retention of a conditioned avoidance response in the rat. Physiol. Behav., 1969, 4: 803-814.
97. Karadzic, V., Kovacevič, R., Momira, D. Sleep in the owl (Strix aluco, Strigidae). In: Sleep 1972: Physiology, Biochemistry, Psy chology, Pharmacology, Clinical Implications. 1st Europ. Congr. Sleep Res., Basel, Karger, 1973: 283-285.
98. Karmos, G. and Martin, J. Motivational aspects of the paradoxical phase of sleep. In: K. Lissak (Ed) Results in Neurophysiology, Neuroendocrinology and Behavior. Budapest, Acad. Kiado. 1969: 106-130.
99. Kety, S.S. The biogenic amines in the central nervous system: The possible role in arousal, emotion and learning. In: F.O. Schmidt (Ed) The Neurosciecnes: Second Study Program. New York, 1970: 324-336.
100. Kety, S.S. The possible role of the adrenergic systems of the cortex in learning. Res. Publ. Ass. Nerv. Ment. Dis., 1972, 50: 376-389.
101. Kitahama, K. Augmentation de sommeil paradoxal apres apprentissage chez le rat. These Lyon: Universite Claude Bernard. 1973.
102. Klein, M., Michel, F., Jouvet, M. Etude polygraphique du sommeil chez les oiseaux. Comptes Rendus des Seances de Biologic (Paris), 1964, 158: 99-103.
103. Koridze, M.G., and Nemsadze, N.D. The effect of paradoxical sleep deprivation on learning. In: Moderne Aspekte der Angewandten und Grundtagenforshung in der Psychophysiologie. Karl-Marx - Universität, Leipzig, 1980, 3: 245-249.
104. Koridze, M.G., Nemsadze, N.D., Maisuradze, L.M. Effect of paradoxical sleep deprivation on the active avoidance in rats. Abstr. VI - the Intern. Neurobiol. Symp. on Learning and Memory, Magdeburg, 1980: 30.
105. Koridze, M.G., Nemsadze, N.D., Maisuradze, L.M., Oniani T.N. Effect of learning on the structure of sleep-wakefulness cycle (Unpublished data).
106. Kovalzon, V.M. and Tsibulsky, V.L. REM deprivation caused by electrical stimulation of the midbrain reticular formation in rats. Fiziol. Zh. SSSR, 1978, 64(8): 1082-1088 (in Russian).
107. Krech, D., Rosenzweig, M.R., Benett, E.L. Environmental impoverishment, social isolation and changes in brain chemistry and anatomy. Physiol. Behav., 1966, I: 99-114.
108. Kruglikov, R.Y., Aleksandrovskaya, M.M., Dish, T.N. Disturbance of the conditioned behavior and morphological changes in the brain of cats during REM deprivation. Zh. Vyssh. Nervn. Deyat., 1975, 25: 471-476 (in Russian).
109. Kruglikov, R.Y., Maizelis, M.L., Brazovskaya, R., Bazian, A.C. On some mechanisms of the disturbance of conditioned: behavior, produced by REM deprivation. Biol. Nauki, 1975, 12: 58-63 (in Russian).
110. Latash, L.P., Danilin, V.P., Manov, G.A., Rait, M.L. On functional interrelations between delta sleep and REM sleep. In: Sleep 1974; Instinct, Neurophysiology, Endocrinology, Episodes, Dreams, Epilepsy, Intracranial Pathology. 2nd Europ. Congr. on Sleep Res., Rome, Karger, Basel, 1975: 356-358.
111. Latash, L.P. and Manov, G.A. Relation of delta-sleep and a physical component of "fast" sleep to the retention and retrieval of verbal material, learned before sleep. Phiziologia Cheloveka, 1970, 1(2): 262-270.
112. Leconte, P. and B1och, V. Effect de la privation de sommeil paradoxal sur l'acquisition et la retention d'un conditionement chez le Rat. J. Physiol. (Paris), 1970, 62: 290.
113. Leconte, P. and Hennevin, E. Sommeil paradoxal et memorisation effet du delai d'endormissement chez le Rat. J. Physiol. (Paris), 1972, 65: 255-256.
114. Leconte, P. and Hennevin, E. Temporal characteristics of the augmentation of PS following learning in rat. Physiol. Behav., 1976, 11: 677-686.
115. Leconte, P. and Hennevin, E. Post-learning PS, reticular activation and noradrenergic activity. Physiol. Behav., 1981, 26: 587-594.
116. Leconte, P., Hennevin, E., Bloch, V. Analyse des effects d'un apprentissage et son niveau d'acquisition sur sommeil paradoxal consecutif. Brain Res., 1973: 49: 367.
117. Levin, S. Stress and Behavior. Sci. Am., 1971, 224: 26-31.
118. Levin, S. and Brush, R.F. Adrenocortical activity and avoidance learning as a function of time after avoidance training. Physiol. Behav., 1967: 385-388.
119. Levin, S. and Gombosh, D. Increase in REM time as a function of the need for divergent thinking. In: Sleep 1972: Physiology, Biochemistry, Psychology, Pharmacology, Clinical Implications. 1st Europ. Congr. Sleep Res., Karger, Basel, 1973: 389-403.
120. Lindbrink, P. The effect of lesions of ascending noradrenaline path ways on sleep and waking in the rat. Brain Res., 1974, 74: 19-40.
121. Linden, E.R., Bern, D., Fishbein, W. Retrograde amnesia: prolonging the fixation phase of memory consolidation by PS deprivation. Physiol. Behav., 1975, 14: 409-412.
122. Lindsley, D.B. Emotion. In: S.E. Stevens (Ed) Handbook of Experimental Psychology. Wiley and Sons, New York, 1951: 473-516.
123. Lucero, M.A. Lengthening of REM sleep duration consolidative to learning in the rat. Brain Res., 1970, 20: 319-322.
124. Lucas, E.A., Powell, E.W., Murphree, O.D. Hippocampal theta in nervous pointer dogs. Physiol. Behav., 1974, 12: 609-613.
125. Mancia, M. Sleep and Instinct: An interdisciplinary approach. In: Sleep 1974: Instinct, Neurophysiology, Endocrinology, Episodes, Dreams, Epilepsy, Intracranial Pathology. 2nd European Congr. on Sleep Res., Rome, Karger, Basel, 1975: 5-13.
126. Manov, G.A. Some Aspects of Interaction of Sleep and Memory. Kand. Dissertatsia, Moscow, 1973 (in Russian).
127. McCarley, R.W. and Hobson, J.A Single neurons activity in cat gigantocellular tegmental field: Selectivity of discharge in desynchronized sleep. Science, 1971, 174: 1250-1252.
128. McDonough, J.H. and Kesner, R.P. Amnesia produced by brief electrical stimulation of amygdala or dorsal hippocampus. J. Comp. Physiol. Psychol., 1971, 77: 171-178.
129. McGaugh, J.L. Impairment and facilitation of memory consolidation. Activ. Nerv. Super., 1972, 14: 64-74.
130. McGaugh, J.L. The search for the memory trace. Ann. N.Y. Acad. Sci., 1973, 193: 112-123.
131. McGaugh, J.L., Zorntrez, S.F., Gold, P.E., Landfield, P.W. Modification of memory system: Some neurobiological aspects. Quart Rev. Biophys., 1972, 5: 163.
132. McGinty, D.J. and Siegel, J.M. Neuronal activity patterns during rapid eye movement sleep: relation to waking patterns. In: R.R. Drucker-Colin and J.L. McGaugh (Eds) Neurobiology of Sleep and Memory. Academic Press, 1977: 133-158.
133. McGrath, M.J. and Cohen, D.B. Sleep facilitation of adaptive waking behavior: A review of the literature. Psychol. Bull., 1978, 85: 24-57.
134. Miller, L., Drew, W.G., Schwartz, G. Effect of REM sleep deprivation on retention of one trial passive avoidance response. Percept. Mot. Skills., 1971, 33: 118.
135. Milner, B. The memory defect in bilateral hippocampal lesion. Psychiat. Res. Rep., 1959, 11: 43-52.
136. Milner, B. Visual recognition and recall after right temporal lobe excision in man. Neuropsychol., 1968, 6: 191-197.
137. Milner, P.M. Physiological Psychology. Holt, Rinehart and Winston, Inc. N.Y., Chicago, San Francisco, Atlanta, Dallas, Montreal, Toronto-London, Sydney, 1970.
138. Moore, J.D., Hayes, Ch., Hicks, R.A. REM sleep deprivation increases preference for novelty in rats. Physiol. Behav., 1979, 5: 975-976.
139. Morales, F.R., Roig, Y.A., Monti, Y.M., Macadar, O., Budelli, R. Septal unit activity and hippocampal EEG during the sleep-wakefulness cycle of the rat. Physiol. Behav., 1971, 6: 563-576.
140. Morden, B., Mitchell, C., Dement, W. Selective REM sleep deprivation and compensation phenomena in the rat. Brain Res., 1967, 5(3): 339-349.
141. Morden, B., Mullins, R., Levins, S., Cohen, H., Dement, W. Effect of REM deprivation on the mating behavior of male rats. Psychophysiol., 1968, 5: 241-242.
142. Morrel, F. Effect of anodal polarization on the firing pattern of single cortical cells. Ann. N. Y. Acad. Sci., 1961, 92: 860-876.
143. Moruzzi, G. The functional significance of sleep with particular regard to the brain mechanisms underlying consciousness. In: J.C. Eccles (Ed) Brain and Conscious Experience. New York, Springer, 1966: 345-388.
144. Muzio, J.N. Alterations in the Normal Human Sleep EEG Patterns and Retention of Meaningful Verbal Material. Diss., Columbia University, 1971.
145. Nachkebia, N.G. and Oniani, T.N. Influence of functional elimination and partial deafferentation of hippocampus on learning and memory. Acta Neurobiol. Exp. (in press).
146. Nemsadze, N.D. Effect of PS on the acquisition of sound discrimination. Bull. Acad. Sci., Georgian SSR, 1978, 92: 429-432.
147. Nemsadze, N.D. Effect of PS deprivation on the acquisition of active avoidance test. Bull. Acad. Sci., Georgian SSR, 1980, 100: 443-449.
148. Nemsadze, N.D., Koridze, M.G., Maisuradze, L.M., Oniani, T.N. Influence of internal PS deprivation on the acquisition of active avoidance learning. (unpublished data).
149. Nemsadze, N.D., Koridze, M. G., Maisuradze, L.M., Oniani, T.N. Effect of PS deprivation on the retention of passive avoidance. (unpublished data).
150. Ogilvie, R. and Broughton, R.J. Sleep deprivation and measures of emotionality in rats. Psychophysiology, 1976, 13: 249-260.
151. Oniani, T.N The correlation between the emotional tension and EEG dynamics in wakefulness-sleep cycle. In: T.N. Oniani (Ed) Neirofiziologia Emotsii i Tsikla Bodrstvovanie-Son. Metsniereba, Tbilisi, 1976, 2: 5-28 (in Russian).
152. Oniani, T.N. On the functional significance of sleep. Acta Neurobiol. Exp., 1977, 37: 223-246.
153. Oniani, T.N. The organization of neuronal activity of the mesodiencephalic structures in different phases of the wakefulness-sleep cycle. In: Sovremennye Problemy Obshchei Fiziologii Vozbudimikh Obrazovanii. Kiev, "Naukova Dumka", 1978: 176-183 (in Russian).
154. Oniani, T.N. Motivational aspects of the paradoxical phases of sleep. In: T.N. Oniani (Ed) Neurophysiology of Emotion and Sleep-Wakefulness Cycle. Metsniereba, Tbilisi, 1979, 3: 131-156.
155. Oniani, T.N. The Integrative Function of the Limbic System. Tbilisi, Metsniereba, 1980 (in Russian).
156. Oniani, T.N. PS and regulation of the motivational processes (unpublished data).
157. Oniani, T.N., Adams, D., Molnar, P., Gvetadze, L.B., Manjavidze, Sh.D. The dynamics of the neuronal activity of the limbic structures in wakefulness-sleep cycle (unpublished data).
158. Oniani, T.N., Adams, D., Molnar, P., Gvetadze, L.B., Manjavidze, Sh.D., Mgaloblishvi, M.M., Korchinski, R., Varazashvili, P.N. Organization of the neuronal activity in limbic structures during wakefulness-sleep cycle (in press).
159. Oniani, T.N., Adams, D., Molnar, P., Manjavidze, Sh., Gvetadze, L., Beradze, G. Mesodiencephalic mechanisms of regulation of the sleep-wakefulness cycle. In: Sleep 1978. 4th Europ. Congr. Sleep Res., Tirgy-Mureš, 1978, Basel, Karger, 1980: 371-374.
160. Oniani, T.N., Gvetadze, L.B., Manjavidze, Sh.D. The dynamics of neuronal activity of the pontine reticular formation in the wakefulness-sleep cycle (unpublished data).
161. Oniani, T., Koridze, M., Mgalob1ishvi1i, M., Kavkasidze, M. Epileptic EEG discharges induced by hippocampal stimulation and periodicity and ratio of different sleep phases. Waking and Sleeping, 1977, 1(4): 401-406.
162. Oniani, T.N., Koridze, M.G., Nemsadze, N.D., Maisuradze, L.M. Effect of PS deprivation on the acquisition of instrumental conditioned reflex (unpublished data).
163. Oniani, T.N., Koridze, M.G., Nemsadze, N.D., Maisuradze, L.M., Gvetadze, L.B. Behavioral, vegetative and electroencephalographic changes during PS deprivation (unpublished data).
164. Oniani, T.N., Koridze, M.G., Nemsadze, N.D., Maisuradze, L.M., Gvetadze, L.B. Effect of PS deprivation (by water tank) on the heart rate, electroencephalogram and wakefulness-sleep cycle (unpublished data).
165. Oniani, T.N., Molnar, P.P., Naneishvili, T.L. The nature of the paradoxical phase of sleep. Neuroscience Translation. Fed. Amer. Soc.Exp. Biol., 1970, 15: 1-7.
166. Ott, T. The role of hippocampal neurotransmitters in the generation of theta rhythm and memory storage. In: T.N. Oniani (Ed) Gagrskie Besedy. Metsniereba, Tbilisi, 1979, 7: 475-479.
167. Owen, M. and Bliss, E.G Sleep loss and cortical excitability. Am. J. Physiol., 1970, 218: 171-173.
168. Parmelee, A.H., Akisama, L., Wenner, W.H., F1esher, J. Activated sleep in premature infants. APSS, March, 1964. (see Mancia, M., 1974).
169. Pearlman, C.A. and Becker, M. Brief posttrial REM sleep deprivation impairs discrimination learning in rats. Physiol. Psychol., 1973, I: 373-376.
170. Pearlman, C.A. and Becker, M. REM sleep deprivation impairs serial reversal and probability maximizing in rats. Physiol. Psychol., 1974, 2: 509-512.
171. Pearlman, C. and Becker, M. REM sleep deprivation impairs bar-press acquisition in rats. Physiol. Behav., 1974, 13: 813-817.
172. Pearl man, C.A. and Greenberg, R. Posttrial REM sleep: A critical period for consolidation of shuttle-box avoidance. Anim. Learn. Behav., 1973, 1: 49-51.
173. Pegram, V., Hammond, D., Bridger, W. Effects of protein synthesis inhibition on sleep in mice. Behav. Biol., 1973, 9: 377-382.
174. Penfield, W. and Milner, B. Memory deficit produced by bilateral lesions in the hippocampal zone. Arch. Neurol. Psychiat., 1958, 79: 475-479.
175. Petre-Quadens, O. Sleep in the human newborn. In: O. Petre-Quadens and J.D. Schlag (Eds) Basic Sleep Mechanisms. New York, London, 1974: 355-376.
176. Petsche, H., Gogolak, G., Stumpf, Ch. Unit firing and the shape of theta waves in the rat's hippocampus. Electroenceph. Clin. Neurophysiol., 1968, 24: 390.
177. Petsche, H., Stumpf, Ch., Gogolak, G. The significance of the rabbit's septum as a relay station between midbrain and the hippocampus. Electroenceph. Clin. Neurophysiol., 1962, 14: 202-211.
178. Plumer, S.J., Mattheus, L., Tucker, M., Cook, T.M. The water tank technique: Avoidance conditioning as a function of water level and pedestal size. Physiol. Behav., 1974, 12: 285-287.
179. Portnoff, G., Backeland, F., Goodenough, D.R. Retention of verbal material perceived immediately prior to onset of NREM sleep. Percept. Motor Skills, 1966, 22: 751-758.
180. Quartermain, D., Paolino, R.M., Miller, N.E. A brief temporal gradient of retrograde amnesia independent of situational change. Science, 1965, 149: 1116-1118.
181. Riccio, D. and Hodges, L. Retrograde amnesia produced by hypothermia in rats. J. Comp. Physiol. Psychol., 1968, 66: 618-622.
182. Roffuerg, H.P., Muzio, J.N., Dement, W.C. Ontogenetic development of the human sleep-dream cycle. Science, 1966, 152: 604-619.
183. Roig, J.A., Budelli, R., Macadar, O., Monti, Y.M. Hippocampal theta rhythm in relation with unit discharges in septum and hippocampus. Electroenceph. Clin. Neurophysiol., 1970, 28: 520.
184. Rojas-Ramirez, Y.A. and Tauber, E.S. PS in two species of avian predators (falconiformes). Science, 1970, 167: 1754-1755.
185. Rojas-Ramirez, Y.A. and Drucker-Colin, R.R. Phylogenetic correlations between sleep and memory. In: R.R. Drucker-Colin and G.L. McGaugh (Eds) Neurobiology of Sleep and Memory. Academic Press, New York, San Francisco, London, 1975: 57-75.
186. Roldan, E., Weiss, T., Fifkova, E. Excitability changes during the sleep cycle in the rat. Electroenceph. Clin. Neurophysiol., 1963, 15: 775-785.
187. Ruckenbusch, J. Active cortical an cours du sommeil chez la cheore. Comptes Rendus des Seances de la Societe de Biologia (Paris). 1962, 156: 867-870.
188. Ruckenbusch, J. Comparative aspects of sleep and wakefulness in farm animals. In: M.H. Chase (Ed) The Sleeping Brain. Los Angeles, 1972: 23-28.
189. Ruckenbusch, J. Sleep deprivation in cattle. Brain Res., 1974, 78: 495-499.
190. Segales, T. and Domino, E.F. Effects of stress and REM sleep deprivation on the patterns of avoidance learning and acetylcholine in the mouse. Psychopharmacologia (Berlin), 1973, 29: 307-315.
191. Sakuma, N. Influence of avoidance conditioning on sleep. Shikoku Acta. Medica, 1976, 32: 339-354.
192. Shinkman, R. and Komfman, K. Time course of retroactive effects of hippocampal stimulation on learning. Exp. Neurol., 1972, 34: 476-483.
193. Shiromani, P., Gutwein, B.M., Fishbein, W. Development of learning and memory in mice after brief PS deprivation. Abstracts, Society for Neurosci., 8th Ann. Meet., St. Louis, Missouri, 1978, 4: 263.
194. Siegel, Y.M. Unit activity in the reticular formation and REM sleep. In: R. Drucker-Colin, M. Shkurovich, M.B. Sterman (Eds) The Functions of Sleep. Acad. Press, N. Y., 1979: 31-71.
195. Siegel, Y.M. Behavioral functions of the reticular formation. Brain Res. Rev., 1979, 1: 69-105.
196. Siegel, Y.M. and McGinty, D.J. Pontine reticular formation neurons: relationship of discharge to motor activity. Science, 1977, 196: 678-680.
197. Siegel, Y.M., McGinty, D.J., Breedlove, S.M. Sleep and waking activity of pontine gigantocellular field neurons. Exp. Neurol., 1977, 53: 553-573.
198. Sloan, M.A. The effect of deprivation of rapid eye movement (REM) sleep on maze learning and aggression in the albino rat. J. Psychiat. Res., 1972, 9: 101-111.
199. Smith, C., Kitahama, K., Valtax, J.L., Jouvet, M. Increased PS in mice during acquisition of a shock avoidance task. Brain Res., 1974, 77: 221-230.
200. Squire, L.R., Smith, G.A., Barondes, S.H. Cycloheximide affects memory within minutes after the onset of learning. Nature (London), 1973, 242: 201-202.
201. Sterman, M.B., Knauss, T., Lehmann, D., Clemente, C.D. Circadian sleep and waking patterns in the laboratory cat. Electroenceph. Clin. Neurophysiol., 1965, 19: 509-517.
202. Stern, W.C. Acquisition impairments following rapid eye movement sleep deprivation in rats. Physiol. Behav., 1971, 7: 345-352.
203. Stern, W.C, Hartmann, E., Draskoczy, P.P., Schildkraut, J.J. Behavioral effects of centrally administered 6-hydroxydopamine. Psychol. Rep., 1972, 30: 815-820.
204. Tauber, E.S., Weitzman, E.D., Korey, S.R. Eye movement during behavioral inactivity in certain bermuda reef fish. Comm. in Behav. Biol., 1969, Part A(3): 131-135.
205.Valtax, J.L., Jouvet, D., Jouvet, M. Evolution electroencephalographique des differents etats de sommeil chez le chaton. Electroenceph. Clin. Neurophysiol., 1964, 17: 218-233.
206. Van Hulsen, Z.J.M. and Coenen, A.M. Selective deprivation of PS and consolidation of shuttle-box avoidance. Physiol. Behav., 1979, 23: 821-826.
207. Van Hu1zen, Z.J.M. and Cofmen, A.M.J. Deprivation of PS and two-way avoidance acquisition. Neurosci. Lett., 1981, 24(7): 483.
208. Van Omer, E.B. Sleep and retention. Psychol. Bull., 1933, 30: 413.
209. Van Twyver, H. Sleep patterns of five rodent species. Physiol. Behav., 1969, 4: 901-906.
210. Van Twyver, H. and Allison, T. Sleep in the opossum, Didelphis marsupialis. Electroenceph. Clin. Neurophysiol., 1970, 29: 181-189.
211. Van Twyver, H. and Allison, T. Sleep in the armadillo, Desypus novemcinctus at moderate and low ambient temperatures. Brain, Behav. and Evol., 1974, 9: 107-120.
212. Vardaris, R.M. and Schwartz, K.E. Retrograde amnesia for passive avoidance produced by stimulation of dorsal hippocampus. Physiol. Behav., 1971, 6: 131-135.
213. Vein, A.M. Wakefulness and Sleep. Moscow, 1970 (in Russian).
214. Vein, A.M. Disturbances of Sleep and Wakefulness. Meditsina, Moscow, 1974 (in Russian).
215. Vertes, R.P. Selective firing of rat pontine gigantocellular neurons during movement and REM sleep. Brain Res., 1977, 128: 146-152.
216. Vimont-Vicary, P., Jouvet-Monnier, D., Delorme, F. Effects EEG et comportementaux des privations de sommeil paradoxal chez le chat. Electroenceph. Clin. Neurophysiol., 1966, 20: 439-449.
217. Vinogradova, O. Hippocampus and Memory. Nauka, Moscow, 1975 (in Russian).
218. Vinogradova, O.S. and Brazhnik, E.S. Neuronal aspects of septo-hippocampal relations. In: Function of the Septo-Hippocampal System. Ciba Found. Symp. 58, Elsevier, Amsterdam, Oxford, New York, 1978: 147-177.
219. Vital-Durand, F. and Michel, F. Effects de la deaferentation peripherique sur le cycle Veille sommeil chez le chat. Arch. Ital. Biol., 1971, 109: 166-186.
220. Vogel, G.W. A review of REM sleep deprivation. Arch. Gen. Psychiat., 1975, 32: 749-761.
221. Webb, W.B. Selective and partial deprivation of sleep. In: Sleep: 1972 Physiol. Biochem. Psychol., Pramacol., Clinica Implicat. 1st Europ. Congr. Sleep Res., Basel, Karger, icns, 1973: 176-184.
222. Webb, W.B. and Agnew, H.W. Sleep stage characteristics of long and short sleepers. Science, 1970, 168: 146-147.
223. Weiss, T. and Roldan, E. Comparative study of sleep cycles in rodents. Experientia, 1964, 20: 286-281.
224. Weitzman, E.D., Kriphe, D.F., Pol1ar, C., Dominguez, J. Cyclic activity in sleep of Macaca mulatta. Arch. Neurology (Clinical), 1965, 12: 463-467.
225. Wetzel, W., Ott, T., Matthies, H. Posttraining hippocampal rhythmic slow activity ("theta"') elicited by septal stimulation improves memory consolidation in rats. Behav. Biol., 1977, 21: 32-40.
226. Yaroush, R., Sullivan, M.J., Ekstrand, B.K. Effect of sleep on memory. II. Differential effect of the first and second half of the night. J. Exp. Psychol., 1971, 80: 361-366.
227. Zimmerman, J., Stoyva, J., Metcalf, D. Distorted visual feed back and augmented REM sleep. Psychophysiology, 1971, 7: 298.