Neurobiology of Sleep-Wakefulness Cycle 2(3-4): 64-74, 2002
Printed in Georgia. All rights reserved.
© 2002 NSWC
TRANSFORMATION OF NEURONAL ACTIVITY OF THE PARIETAL ASSOCIATIVE ZONES OF THE CEREBRAL CORTEX IN SLEEP-WAKEFULNESS CYCLE AND ITS INFORMATIONAL IMPLICATIONS
T. Oniani, L. Gvetadze, Sh. Manjavidze, N.Oniani, M. Eliozishvili
Department of Neurobiology of Sleep-Wakefulness Cycle, I.S. Beritashvili Institute of Physiology, Georgian Academy of Sciences, Tbilisi
Accepted in revised form 20 November 2002; recieved 31 July 2002.
Summary
The dynamics of neuronal activity of the so-called association zones (AZ) of the parietal regions (AZPR) of the cerebral cortex in the sleep-wakefulness cycle (SWC) has been studied in chronic cats. Analysis of the obtained experimental data leads to the following conclusions: 1. Activity of the majority of neurons in the AZPR throughout the SWC undergoes qualitative changes, that manifest themselves in the transformation of their discharge pattern from regular, i.e. evenly distributed in time, type, characteristic of the state of wakefulness, to the cluster-pause type, characteristic of the slow-wave sleep (SWS). And the neuronal activity, typical of wakefulness, is restored in the paradoxical sleep (PS) phase. 2. Against the background of deep SWS (DSWS), the mean frequency of neuronal cluster discharges, in the AZPR of the cortex, is appreciably higher than that in active wakefulness. This fact provides evidence for the postsynaptic inhibition of neurons during pauses, which upon termination, are immediately followed by the rebound of activity and the respective rise in the frequency of neuronal discharges. 3. At DSWS, the mean duration of a pause between the discharge clusters is considerably longer than that of a cluster. 4. A smaller portion of neurons in AZPR of the cortex, exhibits only quantitative changes in SWC, without transforming their activity to the cluster-pause type. In one group of neurons these changes are evidenced by a decrease in the discharge in SWS, as compared with waking and PS, while the other group may reveal an opposite picture, by enhancing their activity just in the phase of SWS. 5. In the AZPR of the cortex there can also be detected such neurons that are activated selectively in one or another SWC phase. 6. Most neurons in the cortex AZ enhance their activity at the change from SWS to PS with the occurrence of single ponto-geniculo-occipital spikes, prior to the appearance of any signs of changes in the electrocorticogram. 7. Most neurons in the cortical AZPR abruptly sometimes down to a full stoppage, reduce their activity immediately after termination of PS, as well as with the occurrence of short episodes of EEG desynchronization without sings of behavioral arousal against the background of DSWS. 8. It is conjectured that the changes in neuronal activity of the AZPR of the cerebral cortex over SWC display the dynamics of cognitive processes, which on their part, are determined just by the transformation (both quantitative and qualitative) of the neuronal activity of the cerebral cortex, in general, and of its AZ, in particular.
Key Words: Neuronal activity, sleep-wakefulness cycle, association zones of the parietal regions of the cerebral cortex.
Introduction
It is commonly believed that the association zones (AZ) of the cerebral cortex play an exceptionally important role in the regulation of higher psychic functions, such as learning, memory, consciousness, etc. (Nobel and Dewson, 1966; Delgado, 1971; Pribram, 1975; Szentagothai, 1969, 1975, 1978; Eccles, 1980). This assertion, alongside with other facts, is supported by the availability of abundant morphological connections both of the specific projection zones of the cortex, and the subcortical structures with the AZ. It can be conjectured that among other integral brain phenomena, the sleep-wakefulness cycle (SWC) is not an exception, in the sense of AZ involvement in its regulation. This assumption is also strongly supported by the fact that it is the psychic activities that undergo the most drastic, and typical changes over SWC. Of these latter, the most outstanding one is the process of consciousness, which declines with the onset of sleep, and is almost completely blocked as the deep slow-wave sleep (DSWS) sets in. On account of the fact that the AZ play an essential part in the regulation of the process of consciousness, investigation into the dynamics of neuronal activity over SWC has particular interest. In this respect, the AZ of the parietal regions (AZPR) deserve special attention. Thus, it is assumed that only the AZPR are most actively involved in the organization of cognitive phenomena, as distinct from the frontal region AZ, that are directly responsible for the regulation of motivational and emotional processes (Nauta, 1964, 1971, 1972).
The dynamics of neuronal activity of the cerebral cortex has been the subject of numerous publications referring to different periods. However, only the experiments that have been carried out after the discovery of paradoxical phase of sleep (PS) are informative. Evarts (1967), while recording the activity of pyramidal neurons of the cerebral cortex against the background of three clearly identified phases of SWC (waking, slow-wave sleep (SWS) and PS) set forth a systemic description of their dynamics over these phases. At the same time, he made a differentiation between the pyramidal neurons with large, and small bodies. The so-called large neurons, displaying the tendency to phasic activation at contralateral movements, were inactivated against the background of relaxed wakefulness and showed a rise in their discharge at frequency deep sleep. As for small neurons, that had shown tonic activity during wakefulness, they decreased their discharge frequency at the transition from wakefulness to sleep. In addition, he described a transformation of the work of pyramidal neurons from regular, i.e. more or less evenly distributed in time, as at wakefulness, to the cluster-pause activity, typical of both of the sleep phases.
While studying the activity of neurons in AZPR of the cerebral hemispheres, Noda and Adey (1970) on cats, and Desiraju (1972) on monkeys revealed a regular decline in their discharge frequency at the change from wakefulness to SWS, and the following restoration of their activity reaching the level typical of active wakefulness during PS. Besides, at active wakefulness and PS neurons of the aforesaid cortical regions were discharging without having changed their activity to the cluster-pause pattern. The authors explained the cases when in PS the AZPR neurons started discharging in clusters alternating with more or less long pauses, by that these neurons were functionally related with the brain motor system and started discharging at high frequencies only in the presence of PS phasic motor components.
Thereafter, Steriade (1978) described the peculiarities of neurons with long- and short axons in the sensorimotor association area of cerebral cortex and confirmed the similarity of wakefulness and PS in the aspect of the dynamics of these two functionally different type of neuronal discharge. The discharge of neurons with long axons during SWS decreases statistically significantly in comparison with other SWC phases.
In carrying out the experiments described below, concerned with elucidating the dynamics of neuronal activity of AZPR of the cerebral cortex, we have been motivated, one the one hand, by a presumed involvement of the cortical AZ in the organization of higher psychic functions of the brain, and on the other hand, by the desire to gain an insight into the dynamics of neuronal activity of these structures over SWC, with regard to its complex structure reflecting different levels of the brain integrative activity.
Methods
The experiments were carried out with 5 pubertal cats. Preparation of the skull for microelectrodes' manipulations and surgery for insertion of steel electrodes in different brain structures was performed under nembutal (35 ml/kg) anaesthesia. A trepanation hole was drilled in the skull, the position of its centre was determined according to the Atlas of Reinoso-Suarez (1961) (Fig. 1). A cylindrical plexiglass thread bush was inserted in the orifice and fixed in position with noracryl, the thread bush being treated on the inner surface. In order to limit pulsation, the bottom of the thread bush was covered with a plastic film ("Parafilm"). With a view to studying the dynamics of AZPR neuronal activity discharges of individual neurons have been recorded against the background of different cycles. In order to identify the various SWC phases we have recorded: the electroneocorticogram, electrohippocampogram, the electrical activity of the mesencephalic areas from where ponto-geniculo-occipital (PGO) spikes were generated, as well as the electromyogram from cervical muscles, and eye movements. The neuronal discharges of cortical AZPR have been recorded against the background of: active wakefulness (desynchronized electroneocorticogram; well-pronounced hippocampal theta-rhythm; no PGO spikes; markedly enhanced activity of cervical muscles; no rapid eye movements (REMs)), passive wakefulness (desynchronized electroneocorticogram with occasional episodes of spindle-like activity; irregular and meager hippocampal theta-rhythm; no PGO spikes or REMs; well-pronounced electromyogram), light SWS (LSWS) (the electroneocorticogram is slightly synchronized; the hippocampal theta-rhythm is disturbed and instead there occur high-amplitude slow potentials; no PGO spikes or REMs the electromyogram is markedly weakened), DSWS (with high amplitude slow potentials prevailing all over the range of delta-rhythm; PGO spikes occur only in the final period of the phases; no REMs; the electromyogram is either considerably inhibited or is not in evidence at all) and PS (the electroneocorticogram is desynchronized; the hippocampal theta-rhythm is well pronounced; the frequency of PGO spikes, that start occurring in clusters, is markedly increased; and there appear REMs.
The microelectrodes, made of tungsten wire with the diameter of 200 to 250 µm, were sharpened electrolytically. Their entire surface, excepting the tip, was insulated by a special varnish. Resistance of the electrodes equaled 5 to 10 Mom. The microelectrodes were inserted in the brain with the aid of a manipulator fixed in the thread bush. One step of the micromanipulator shifted the microelectrode by 50 µm.
Extracellular action potentials of neurons led from gyrus lateralis and gyrus suprasilvius through a miniature cathode follower were fed into the amplifiers of a double oscillograph. The neuronal discharges were recorded on a photofilm from the screen of the oscillograph, as well as on a magnetic tape and (following the amplitude discrimination), were read out on paper, in parallel with electroencephalograms. Recordings on the magnetic tape were analysed by means of a microcomputer. A multichannel discriminator permitted to identify the activity of a single neuron, or of several neurons, at recording multineuronal activity, however, the activity of only one neuron, with the highest amplitude peak, on the paper was read out. While plotting the histograms, impulse sequences of each neuron, isolated by the discriminator were processed. To avoid errors induced by noise, the activity of neurons with the lowest amplitude peaks was not analysed. The mean frequency values of impulse activity, standard deviations, and the significance of the observed changes were calculated by Student's t-test.
While making the quantitative analysis of the neuronal activity of AZPR we took account of the fact that even in one and the same SWC phase it may vary over a wide range, which accounts for the numerous short intervals of several, to dozens of, seconds that considerably differ from each other in their discharge frequency. Therefore, for constructing the histograms we chose fairly long intervals (2.5 to 3 min) from various periods of one and the same phase, which provided us with a general picture of the discharge frequencies at each physiological state.
While analysing data on the dynamics of neuronal activity of cortical AZPR, apart from quantitative assessment of the mean discharge frequency at various SWC phases, special consideration was given to certain parameters of qualitative transformation of the activity from regular to a cluster-pause pattern. In particular, we determined the mean discharge frequency in clusters, as well as the temporal correlation of clusters and pauses against the background of SWS.
Results and Discusion
The most typical picture of dynamics of a single unit activity in cortical AZPR is presented in Fig. 2. As seen in the active wakefulness, neuronal changes are more or less evenly distributed in time (Fig. 2A). However, as SWS gradually sets in, initially there occur quantitative changes in neuronal discharge frequency, expressed in its decrease (Fig. 2B, First half of the record), and it is only later that the pattern of activity gets altered, i.e. there occur qualitative changes. These changes manifest themselves in the transition of the activity, that is from evenly distributed in time, to the cluster-pause pattern (Fig. 2B, Second half). This latter type of activity gets best pronounced with the onset of DSWS (Fig. 2C) and persists throughout the entire course of the given phase. With the nearing of PS phase onset, testified by the occurrence of single PGO spikes, as well as by the development of complete atony of the cervical muscle, the cluster-pause type activity gets gradually deranged, and, against the background of PS, it resembles the neuronal activity typical of active wakefulness (Fig. 2D).
While studying quantitative changes in the neuronal activity of the brain as a whole, and of cortical AZPR in particular, over SWC phases, it is important to identify different levels of wakefulness as well as the sleep phases. Fig. 3 demonstrates statistic results of quantitative changes in the neuronal activity of the lateral gyrus at various phases and stages of the SWC. It is quite clearly seen that if the neuronal activity of the lateral gyrus is considerably elevated at DSWS and PS, in contrast to passive wakefulness, then there occurs rather a different picture when active wakefulness background recording is intended to be compared with. In this case the highest discharge frequency can be viewed against the background of active wakefulness and PS, though a total activity is noticeably lowered even at DSWS, despite a fairly higher frequency of cluster discharges.
This fact can certainly be explained by a predomination, in time, of pauses over clusters. Very often, however, though there are clearly expressed pauses between the clusters at DSWS, the increase in the average cluster amount is so important that a total activity of single neurons of cortical AZPR exceeds their activity against the background of active wakefulness and PS. Fig. 4 represents the histogram-like data illustrating the dynamics of the discharges of two neurons, registered from the lateral gyrus in the SWC.
It can be seen that with the onset of SWS, if one neuron significantly brings its activity down as compared to the wakefulness, and especially, against the background of PS, the other one is discharging at a comparatively lower rhythm and, on the contrary, gets more activated, most of all against the background of SWS. However, it should be noted that neurons of the second type are rarer than those of type one.
An interesting picture can also be observed in those cases when the activities of two or three neurons of the cortical AZPR are tracked through one and the same recording microelectrode. Fig. 5 shows the activity dynamics of two neurons of lateral gyrus. As demonstrated, their discharges are more or less evenly distributed in time against the background of active wakefulness (Fig. 5A), whereas in passive wakefulness (Fig. 5B), similar to LSWS (Fig. 5C), both of the neurons display the cluster-pause activity. However, their active episodes and pauses are asynchronous, as a result, the cluster activity of one of the neurons coincides with the pause of the other. With the change to DSWS, active and silent episodes of both neurons set in synchronously (Fig. 5D, E), but with the development of PS, the activity type which is characteristic of active wakefulness is restored (Fig. 5F). The similar picture could be found looking more attentively through the recordings of the dynamics of multineuronal activity of motor cortex (Grentzfeld and Jung, 1961) and Gyrus Cingulus of cat's brain (Manjavidze, et al. 1988).
In other cases the change in the neuronal activity is mainly manifested by the quantitative, rather than qualitative, shifts. An illustrative example is given in Fig. 6, involving registration of two neurons, whose spikes differ from each other in their amplitude. As demonstrated, discharges of both neurons were more or less evenly distributed in time against the background of active wakefulness (Fig. 6A), while in passive wakefulness (Fig. 6B) their discharge frequency decreased drastically, without being transformed into the cluster-pause activity. A clear-cut cluster type activity was not in evidence even during DSWS (Fig. 6C, D). In PS the activity of either neuron got enhanced, reaching the level typical of active wakefulness. Consider also that the highest discharge frequency was observed at the so-called emotional stage of PS (Fig. 6E), whereas in the non-emotional stage the discharge frequency decreased considerably (Fig. 6F). Attention is also drawn to the fact that in the PS emotional stage, there appeared discharges of a third neuron (with a small spike amplitude) whose activity was hardly traceable under other sleep phases or stages. Such selective activation of some AZPR neurons in specific SWC phases, though not very frequently, but is still observable.
Fig. 7 illustrates a curious example of behavior of three neurons recorded through the same microelectrode from the AZPR. As can be seen, under active wakefulness all the three neurons discharge at more or less regular pace. However, in the other SWC phases that follow, there occur disparities in their behavior. Activity of the neuron with the greatest spike amplitude in DSWS, undergoes qualitative changes, i.e. the neurons start to discharge in the cluster-type manner. Despite fairly lengthy pauses between the clusters, owing to the drastic rise in the mean number of discharges in clusters, the level of the overall activity in DSWS tangibly exceeds the one observed at passive or active wakefulness. As for the other two neurons, they display only quantitative changes, i.e. their discharge frequency decreases over passive wakefulness and DSWS, as compared with active wakefulness, and with PS in particular. An obvious difference in the behavior of the three neurons is that the neuron with the highest spike amplitude almost stops discharging at PS (Fig. 7E), while the remaining two neurons enhance their activity to the level even excelling the one corresponding to active wakefulness.
As indicated above, apart from analyzing the quantitative and as well as qualitative, changes in the neuronal activity in AZPR, we also focused on studying such parameters as: variation of the mean values of neuronal discharges in clusters over SWS phase.
Fig. 2C and 3F demonstrate a clear-cut increase in the mean frequency of discharges in clusters over SWS, as compared with active wakefulness and PS. Very often, the increase in the mean frequency of neuronal discharges in clusters against the background of DSWS is so significant that it results in the considerable enhancement of the neuronal activity over SWS, as compared both to active wakefulness and PS.
It should be noted that the cluster-pause activity of neurons of the AZPR is exclusively typical of SWS, and by no means of PS. The reason why we focus on this fact is that after Evarts's classical works (1962) bearing on the neuronal activity of the brain over SWC, it became habitual to think that the cluster-pause neuronal activity was typical of both SWS and PS, though in this latter, discharge clusters, as well as pauses, might be longer. Later on quite a few scholars have reported that neurons of cerebral cortex, as well as of other brain structures (Desiraju, 1972; Noda and Adey, 1973, 1975; Mukhametov and Strokova, 1976; Manjavidze, et al. 1988; Varazashvili, 1988) discharge at regular intervals in PS, i.e. their discharges are more or less evenly distributed in time. But nevertheless, at considering the general mechanisms of brain work over SWC, many authors typically refer to Evart's original publications (see Eccles, 1980). And the data described therein, presumably bear only on motor neurons whose start discharging in groups of clusters in response to the development of the phasic motor components of PS, thereby giving an impression, that the neuronal activity proceeds according to the cluster-pause pattern. In our experiments, not in a single case, did the neuronal activity of cortical AZPR change form cluster-pause pattern to more or less regular discharges at the transition from SWS to PS, as characteristic of active wakefulness. A true cluster-pause activity developed only against the background of SWS. Though, not infrequently, in AZPR there can be detected such neurons whose discharges in PS correlate with the phasic components of this particular physiological process, such as eye movements, isolated contraction of the somatic muscles, accompanied by limb movements or vibris (Fig. 8). In all probability, such distribution pattern of neuronal discharges is qualitatively different from the cluster-pause activity, typical of SWS. This contention is also strongly supported by the fact that at recording the activities of two neurons in the lateral gyrus during PS through one and the same electrode during PS, only one of them, with a larger spike amplitude, was discharging in clusters, while the other, with a smaller spike amplitude, continued to display regular excitation. As for SWS, in this phase clusters and pauses in discharges of both the neurons develop synchronously, which is indicative of the alternation of active episodes and inhibition, rather than of the occurrence of discharge clusters in response to the development of the phasic motor components of PS. In this respect, it is also informative that over the so-called non-emotional stage of PS, in the absence of clear-cut phasic motor components, activity of the neuron with the larger spike amplitude does not only follow the cluster-pause pattern but is even decreased to zero (Fig. 8). This also testifies to that the occurrence of cluster discharges in the described phase is related to the motor phasic components of PS.
At studying the dynamics of various neurophysiological parameters over SWC, one can readily identify the clear-cut transition moments from one phase to another. In this connection, it is also of interest to run down the dynamics of the neuronal activity in cortical AZPR at the change from SWS to PS, and from PS to wakefulness or SWS. Logically, in the sequence of SWC phases, SWS, as a rule, should be followed by PS, however, this regularity is often broken, presumably for various reasons, and before PS sets in, against the background of SWS there may appear more or less long episodes of wakefulness, which occasionally develop into a full-fledged phase, judging from all the behavioral and EEG parameters.
The dynamics of neuronal activity of cortical AZPR during the development of episodes of wakefulness against the background of SWS, as well as at the changes from SWS to PS, and from PS to a subsequent phase, is presented in Tables 1 and 2. At considering the Tables, attention is drawn to the following facts: 1. Throughout the development of comparatively short episodes (less than 10 sec) of EEG desynchronization against the background of SWS without behavioral arousal, i.e. with no visible motor responses, most neurons of both the cortical AZPR drastically reduce their activity, sometimes to zero, whereas at longer episodes (more than 10 sec) of EEG desynchronization, in the presence of weak or full-fledged behavioral responses, the initial inhibition of discharges is followed by their more or less complete restoration. 2. At the change from DSWS to PS the neuronal activity in the two gyri under study differed from each other significantly. Thus, for example, most neurons in the lateral gyrus showed activation as early as before the electorcorticogram (ECG) desynchronization, while in the suprasylvian gyrus, the enhancement in the activity of most neurons coincided with the occurrence of ECG desynchronization. Besides, it was only in the suprasylvian gyrus that we detected single neurons whose activity got inhibited at the transition from DSWS to PS, however, later on with the development of a full-fledged PS phase, they got activated again. 3. Most neurons of AZPR are activated over PS, as compared to SWS, and the highest frequency in their discharges coincides in time with REMs or other motor components of PS. 4. In both gyri the neuronal activity at the spontaneous discontinuation of PS, i.e. at passing to more or less long episodes of wakefulness, reduces drastically, sometimes to full stoppage of discharges, and restores only if EEG desynchronization is attended by behavioral signs of arousal. But if brief EEG desynchronization episodes are followed by SWS restoration, the corresponding neuronal activity is also recovered.
Conclusions and General Discussion
The above data can be viewed in various aspects, of which the most important seem the following: 1. Assessment of the implications of qualitative and quantitative changes in neuronal activity for SWC regulation with regard to the role of the AZPR of the cortex in the integral brain work as a whole. 2. The informative significance of the dynamics of AZPR neuronal activity for understanding the regularities of transition of one SWC phase to another, and 3. Assessment of the obtained data with a view to gaining better insight into the nature and functional role of individual SWC phases in particular, and of the whole SWC, in general.
The above described data clearly indicate that at the alternation of SWC phases, the activities of most neurons in cortical AZPR undergo qualitative changes that manifest themselves in the transformation of the discharge pattern: the more or less evenly distributed activity, as in active wakefulness and PS, is replaced by the cluster-pause pattern at the change to SWS.
With regard to this circumstance, three facts are to be noted: 1. In terms of time, during DSWS, pauses prevail over clusters. 2. The mean discharge frequency of neurons in cortical AZPR is drastically increased in clusters, as compared even with active wakefulness and PS, which apparently is due to the rebound of excitation following each pause corresponding to the development of active postsynaptic inhibition of the recorded neuronal activity. 3. Despite the presence of fairly long pauses between the clusters of neuronal discharges in cortical AZPR, the overall mean frequency of excitation of neurons over SWS is no less, or even greater than that at waking or PS. It can be conjectured from the above that the cluster-pause type activity of neurons in SWS provides for optimum background first, for the work of neurons without further fatigue, and second, for the fulfillment of SWS functions, in terms of shifting the brain integrative activity to another level.
With account for the role of cortical AZPR in the regulation of general psycho-physiological processes, it can be assumed that the indicated transformations in the activity of neurons may bring about the shift of brain work from the conscious level, typical of waking, to subconscious level typical of DSWS. This by no means implies that the extensive brain structures whose neuronal activity changes from regular to the cluster-pause type at passing from wakefulness to SWS, are involved to an equal extent in the dynamics of consciousness throughout SWC. The cluster-pause activity, when alternation of excitation and inhibition becomes a regularity, provides optimum background for the activity of different neurons and ensembles of neurons and for their involvement in the organization of SWC - which has a very complex function (see Steriade and Amzica, 1998). It is but natural that such change in the activity mode of neurons of cortical AZ may contribute to the transition of psychic processes from one level to another, since it is just these structures that should be involved in the regulation of higher psychic processes.
Thus, despite the occurrence of lengthy episodes of inter-cluster silence between the clusters of discharges, the overall level of neuronal activity in cortical AZPR, can by no means diminish all along DSWS, however, the qualitative transformation of the activity still provides for the adaptive blockage of the process of consciousness and for the recess of neurons after considerable psychic tension. And in PS, alongside with the restoration of the activity typical of active wakefulness, there occurs an off-beat restoration of psychic processes. Presumably, the endogenous stimuli generated under the influence of a specific need in PS at the subcortical level, tone up the cortical neurons so strongly that their activity even exceeds the level of active wakefulness, and often reaches the level corresponding to affective wakefulness.
Part of the neurons in cortical AZPR undergo qualitative changes over SWC, that are manifested in a considerable decline of their discharge frequency with the onset of, and throughout SWS. As PS sets in, their activity returns to or even exceeds the level of active wakefulness. Some neurons (comparatively few in number) of these brain regions alter their activity so largely, in terms of quantity, from phase to phase over SWC, that they almost stop discharging at one phase, and re-burst all of a sudden in the subsequent one. It can be assumed that the like changes in the neuronal activity of cortical AZPR are also contributive to the transition of the brain work from conscious to subconscious level.
Essential to the understanding of functional implications of SWC as a whole, is elucidation of the level of coordinated brain activity not only in clear-cut phases, but also at transition moments from one phase to another. As indicated by certain physiological signs, the change from wakefulness to SWS, and from SWS to PS is comparatively swift, with the respective mechanisms being gradually involved into the process, whereas PS, at least in wild animals and rodents, terminates abruptly to be replaced by more or less long episodes of waking, after which SWS is restored, again in a gradual manner. Investigation into the neuronal activity of AZPR of the cerebral hemispheres furnishes additional information on the transitions of one SWC phase to another, as well as on the dynamics of coordinated brain activity at these transitions. As has been shown, with the gradual development of SWS, most neurons in these structures change their discharge pattern from more or less evenly distributed in time, to the cluster-pause type. However, the pauses are formed gradually and start to display a regular character only against the background of DSWS. It is where discharges get tightly packed in clusters, due to their increased frequency, which in all probability indicates the presence of the phenomenon of rebound to follow active inhibition. Besides, in transition states from active wakefulness to DSWS, the formation of cluster-pause activity even in neurons pertaining to the same locus goes asynchronously, and becomes synchronous only against the background of well-pronounced SWS. Thus, judging by the parameters indicated above, cortical neurons of AZPR work synchronously in three basic conditions of SWC - in active wakefulness, DSWS and PS. As for transitions from one phase to another, neuronal activity thereover is typically asynchronous and irregular. This circumstance, in its turn, is provided informative on the dynamics of the level of coordinated brain activity over SWC. The highest level should correspond to the greatest synchrony in the work of activated neurons. These data shows, that the so-called ECG desynchronization, typical of active wakefulness and PS, is related not to the asynchronous activity of cortical neurons, but to their regular, evenly distributed-in-time synchronous activity. But lying in the basis of the EEG synchronization phenomenon is the transition of evenly distributed neuronal activity into cluster-pause type activity.
With a view to studying the transition moments from one SWC to another, of obvious interest is that, as a rule, PS is terminated abruptly, without a swift development into another phase; as well as the fact that at these moments cortical neurons of AZPR decrease their activity sharply and momentarily. As has been shown, these neurons behave in the similar manner when episodes of wakefulness develop in the form of ECG desynchronization without behavioural arousal against the background of SWS. Gaining an insight into the mechanisms of this phenomenon calls for further experimentation.
References
Delgado, I. Brain and Consciousness. Moscow, 1971, (in Russian).
Desiraju, T. Transformations of discharges of neurons of parietal assiliation cortex during sleep and wakefulness in monkey. J. Neurophysiol., 1972, 35, 3: 326-332.
Eccles, I.C. The Human Psych., Springer International, 1980.
Evarts, E.V. Activity of neurons in visual cortex of the cat during sleep with low voltage fast EEG activity. J. Neurophysiol., 1962, 25: 812-816.
Evarts, E.V. Temporal patterns of discharge of pyramidal tract neurons during sleep and waking in the monkey. J. Neurophysiol., 1964, 27: 152-171.
Evarts, E.V. Unit activity in sleep and wakefulness. In: G.C. Quartion, T. Melnechuk and F.O. Schmitt (Eds.) The Neurosciences. New York, Rockfeller University Press, 1967: 545-556.
Greutzfeldt, O.D. and Jung, R. Neuronal discharge in the cat's motor cortex during sleep and arousal. In: G.E.W. Wolstenholme and M. O'Conor (Eds.) The Nature of Sleep. London, Churchill, 1961: 129-170.
Manjavidze, Sh.D., Gvetadze, L.B., Oniani, T.N., Varazashvili, P.N. The organization of the neuronal activity of the cingulate gyrus in the sleep-wakefulness cycle. In: T.N. Oniani (Ed.) Neurobiology of Sleep-Wakefulness Cycle. Tbilisi, Metsniereba, 1988.
Mountcastle, V.B., Lunch, J.C., Georyopulus, A., Sakata, H., Acuna, C. Posterior parietal association cortex of the monkey: Command functions for operation within extrapersonal space. J. Neurophysiol., 1975, 38: 871-908.
Mukhametov, L.M. and Strokova, N.G. Visual cortex neuronal activity in the sleep-wakefulness cycle. J. Neurophysiologia, 1976, 8: 343-350, (in Russian).
Nauta, N.J.H. Some afferent connections of the prefrontal cortex in the monkey. In: J.M. Warren and K. Akert (Eds.) The Frontal Granular cortex and Behavior. New York, McGraw-Hill, 1964.
Nauta, N.J.H. The problem of the frontal lobe: a preinterpretation. J. Physiol. Rev., 1971, 8: 167.
Nauta, N.J.H. Neural association of the frontal cortex. Acta Neurobiol. Exp., 1972, 32: 125.
Nobel, K. N. and Dewson, J.H. III. A corticofugal projection from insular and temporal cortex to the homolateral inferior colliculus in cat. J. Arch. Research, 1966, 6: 67-75.
Noda, H. and Adey, W.R. Firing of neuron pairs in cat assoliation cortex during sleep and wakefulness. J. Neurophysiol., 1970, 33: 672-684.
Noda, H. and Adey, W.R. Neuronal activity in the association cortex of the cat during sleep, wakefulness and Anesthesia. Brain Research, 1973, 54: 243-259.
Pribram, K.H. Languages of the Brain. Moscow, 1975, (in Russian).
Reinizo-Suarez F. Topographischer Hirnatlas der Katze fur experimental-physiologische Untersuchungen. E. Muck, Darmstadt, 1961.
Steriade, M. Cortical long-axonal cells and possible interneurons during sleep-wakefulness cycle. Behavioral and Brain Sciences, 1978, 3: 465-514.
Steriade, M. and Amzica, F. Coalescence of sleep rhythms and their chronology in corticothalamic networks. Sleep Research Online, 1998, 1(1): 1-10.
Szentagothai, J. Architecture of the cerebral cortex. In: H.H. Jasper, A.A. Nard, Jr. and A. Pope (Eds.) Basic Mechanisms of the Epilepsies. Boston, Little, Brown, 1969: 13-28.
Szentagothai, J. The "module concept" in cerebral cortex architecture. Brain Res., 1975, 95: 475-496.
Szentagothai, J. The neuron network of the cerebral cortex: A functional interpretation. Proc. R. Soc. Lond., 1978, B 201: 219-248.
Varazashvili, P.N. Neuronal activity of some cortical areas of the brain during the sleep-wake cycle. In: T.N. Oniani (Ed.) Neurobiology of Sleep-Wakefulness Cycle. Tbilisi, Metsniereba, 1988.
Tables
| EEG arousal during SWS |
| Short-lasting (< 10 s) |
More durable (> 10 s) |
| activity decreases |
activity does not alter |
activity increases |
activity decreases |
activity first decreases, then restores |
| 32 |
24 |
8 |
16 |
16 |
| SWS→PS |
| activity decreases |
activity decreases with a subsequent activation in PS |
activity remains unaltered |
activated |
| before the onset of EEG desynchronization |
with the onset of EEG desynchronization |
| 8 |
8 |
8 |
16 |
32 |
| PS |
| activity decreases |
activity remains unaltered |
activity increases |
activity gets particularly elevated during phasec components |
| – |
12 |
28 |
20 |
| Emergence from PS (PS→W) |
| activity decreases |
activity decreases, then restores during wakefulness |
activity remains unaltered |
activity increases |
| 56 |
32 |
- |
- |
Table 1. Dynamics of neuronal activity in the lateral gyrus during brief episodes of EEG desynchronization against the background of SWS at the transition from one SWC phase to another.
| EEG arousal during SWC |
| Short-lasting (< 10 s) |
More durable (> 10 s) |
| activity decreases |
activity remains unaltered |
activity increases |
activity decreases |
activity first decreases, then restores |
| 56 |
8 |
- |
32 |
24 |
| SWS→PS |
| activity decreases |
activity decreases with a subsequent activation in PS |
activity remains unaltered |
activity increases before the onset of EEG desynchronization and in PS |
| - |
- |
32 |
56 |
| PS |
| activity decreases |
activity remains unaltered |
activity increases |
activity gets particularly elevated during of EEG desynchronization and in PS |
| 8 |
- |
52 |
50 |
| Emergence from PS (PS→W) |
| activity decreases |
activity decreases, then recovers during W |
activity remains unaltered |
activity increases |
| 88 |
24 |
- |
- |
Table 2. Dynamics of g-SS neuronal activity during brief EEG desynchronization episodes against the background of SWS and at the transition of one SWC phase to another.
Figures
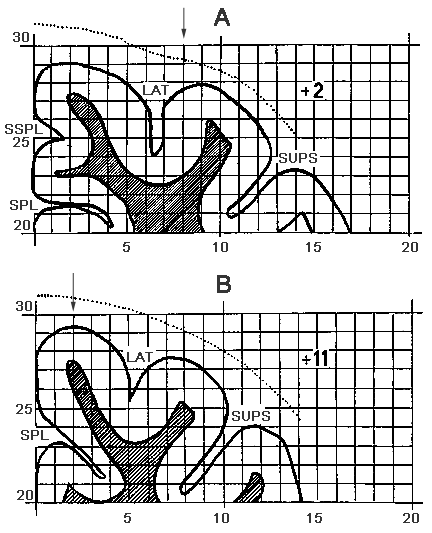
Figure 1. A schematic drawing of the cat brain frontal sections.
A - AP+2, B - AP+11
LAT - G. Lateralis; SUPS - G. Suprasilvius.
Designations: the microelectrode track is indicated by arrows.
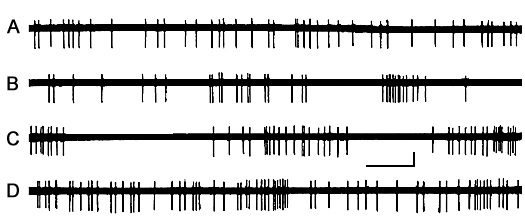
Figure 2. The character of dynamics of single unit discharges AZPR in different SWC phases.
A - active wakefulness, B - LSWS (first half of the record) and well-developed SWS (second half of the record), C - DSWS and D - PS in the presence of mean frequency REM.
Calibration - time 100 ms, amplitude - 100 µV.
Figure 3. The results of statistical treatment of data (by Student) and dynamics of activity of 10 typical AZPR neurons in various phases and stages (within phases) of SWC.
A - active wakefulness, B - passive wakefulness, C - LSWS, D - DSWS, E - PS and F - frequency in a cluster during DSWS.
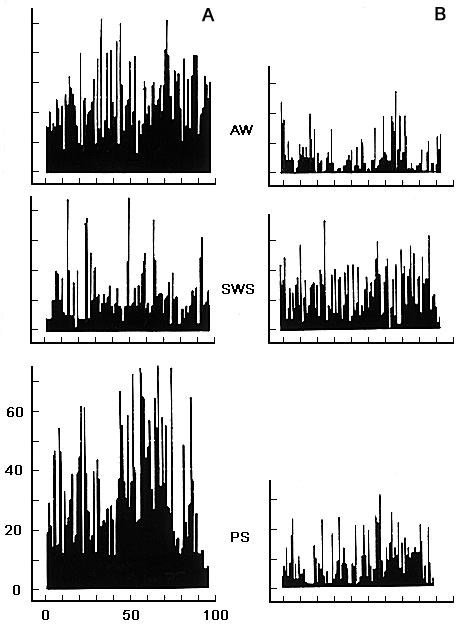
Figure 4. Frequency histograms of neuronal activity in the cortical association zones (gyrus lateralis) in SWC.
On the axis of abscissa - time in sec, on the axis of ordinate - frequency in sec.
Designations: AW - active wakefulness, SWS - slow wave sleep, PS - paradoxical phase of sleep.
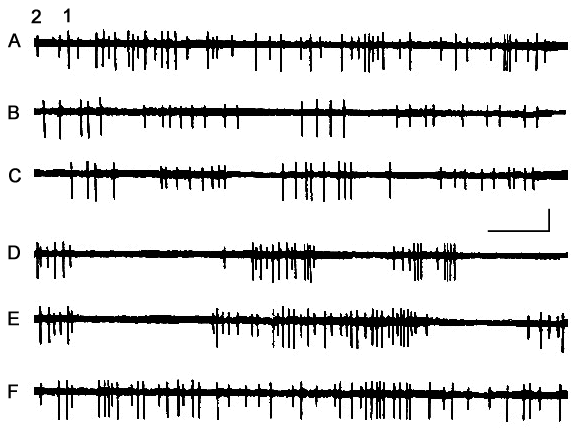
Figure 5. Dynamics of activity of two neurons led through one and the same microelectrode from AZPR in the SWC.
A - active wakefulness, B - passive wakefulness, C - LSWS, D and E - DSWS and F - PS.
Calibration - time 100 ms, amplitude - 100 µV.
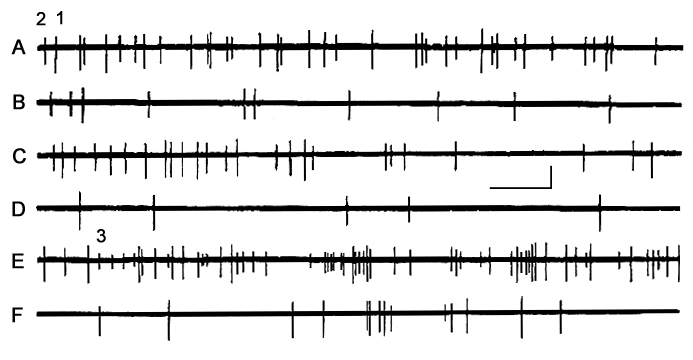
Figure 6. Dynamics of activity of three neurons recorded through one and the same microelectrode from the suprasylvian gyrus in different phases of SWC.
A - active wakefulness, B - passive wakefulness, C, D - DSWS, E - emotional stage of PS and F - nonemotional stage of PS.
Calibration - time 100 ms, amplitude - 100 µV.
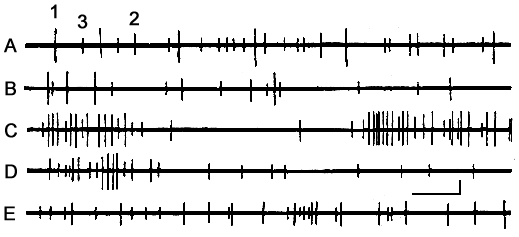
Figure 7. Dynamics of activity of three neurons recorded through one and the same microelectrode from the cortical association zone in SWC.
A - active wakefulness, B - passive wakefulness, C - SWS, D - at the transition from SWS to PS and F - against the background of PS.
Calibration - time 100 ms, amplitude - 100 µV.
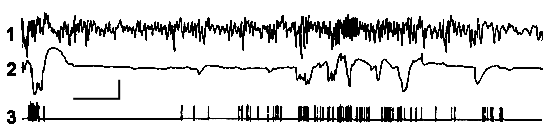
Figure 8. Paper record of electrohippocampogram (1), electrooculogram (2) and activity of a neuron (3) on the background of PS. It is seen that discharges of neurons on the face of REMs.
Calibration - time 5 s, amplitude - 100 µV.
Correspondence: Oniani Tengiz, Prof.,
Department of Neurobiology of Sleep-Wakefulness Cycle,
I.S. Beritashvili Institute of Physiology, Georgian Academy of Sciences,
14, L. Gotua str., Tbilisi, 380060, Georgia.
E-mail: nswc@neurobiology.ge