Neurobiology of Sleep-Wakefulness Cycle 2(3-4): 60-63, 2002
Printed in Georgia. All rights reserved.
© 2002 NSWC
SLEEP-WAKEFULNESS RHYTHM IN CHILD PATIENTS WITH WEST AND LENNOX SYNDROMES.
Department of Pediatrics, Tokyo Medical and Dental University , School of Medicine, 1-5-45 Yushima, Bunkyo-ku, Tokyo, 113-8519, Japan
Accepted in revised form 23 October 2002; recieved 16 March 2002.
Summary
It is generally accepted that epileptic seizures affect the sleep-wakefulness rhythm, and that the sleep-wakefulness rhythm is often disturbed in patients with intractable epilepsy. West syndrome (WS) and Lennox syndrome (LS) are representative catastrophic types of epilepsy in young children, but few studies have described the sleep-wakefulness rhythm of patients with WS or LS. Thus, we examined the chronological alteration of the sleep-wakefulness rhythm in these disorders in 12 patients [WS (n=6), LS (n=6)] split into two groups, benign; n=4, and intractable; n=8. Caregivers recorded the sleep-wakefulness rhythm of the patients daily for at least three years. This data was compared with records obtained from age-matched patients with epilepsies other than WS or LS (control group). Disturbances of the sleep-wakefulness rhythms of patients in the benign group became indistinguishable from the age-matched controls, although this took 2 to 4 years after epileptic seizures ceased. In contrast, the sleep-wakefulness rhythms of patients in the intractable group were different from the controls during the observation period. The disturbance of the sleep-wakefulness rhythm appeared to be more severe in patients with WS or LS than in other child epilepsy patients. The neuronal background of this putative disturbance was discussed.
Key Words: Sleep-wakefulness rhythm, West syndrome, Lennox syndrome, brainstem, monoaminergic system, biological clock.
Introduction
In healthy children, the sleep-wakefulness rhythm develops from the neonatal polyphasic pattern into the monophasic pattern by early childhood. In contrast, in neurologically ill children, the development of this rhythm formation is sometimes disturbed, showing irregular onset of night sleep, a backward shift of the rhythm, and longer duration and frequency of daytime sleep (Okawa and Sasaki, 1987). The disturbance of the sleep-wakefulness rhythm has also been reported in child patients with intractable epilepsy (Konishi, et al. 1995; Jan and O'Donnell, 1996).
West syndrome (WS) and Lennox syndrome (LS) are representative catastrophic types of epilepsy seen in young children (Campos-Castello, 2001). Some patients with WS change into LS, so that some LS patients have WS histories (Ohtahara, 1984). WS and LS might share the common underlying neurological abnormality. As few studies have described the sleep-wakefulness rhythm of patients with WS and LS (Méndez and Radtke, 2001), we examined the chronological alteration of the sleep-wakefulness rhythm of patients with WS or LS.
Methods
Twelve child patients with WS (n=6) or LS (n=6) were the subjects of this study. They were divided into two groups (benign and intractable groups) according to the prognosis of their seizures (Table 1). In the benign group (n=4, WS1-3, LS1), seizures were easily controlled and had not recurred for more than a 1-year period during the time of our observation. In the intractable group (n=8, WS4-6, LS2-6), seizures were difficult to be controlled by various treatments including corticosteroids, ACTH, and ketogenic diet, and recurred within 1 year after any transient remission of seizures. All patients received treatments of 1 to 4 anticonvulsants such as sodium valproate, nitrazepam, clonazepam, diphenylhydantoin, phenobarbital or carbamazepin throughout the study.
For at least three years since they first visited our hospital, each patient's caregiver recorded the daily sleep-wakefulness rhythm (sleep log). Sleep-wakefulness rhythm was also recorded in 127 children with well-controlled epilepsies, other than WS and LS, such as grand mal, absence and partial motor seizure and who were receiving anticonvulsants. The records of sleep wakefulness rhythms of these non-WS or non-LS epileptic patients after they experienced their last seizure were used as age-matched control data.
Sleep-wakefulness rhythm was evaluated from six aspects; nocturnal sleep onset time, morning waking time, nocturnal sleep duration, daytime sleep duration, total daily sleep duration, and the frequency of daytime sleep. If the patient value was beyond the mean±two fold of the standard deviation of the age-matched control value for more than 1 month during a year, that aspect was scored as 1 for the year. A total score for the year was obtained by summing scores of the six above-mentioned monitored aspects. Scores for the year for each patient ranged from 0 to 6.
Results
The benign group (Fig. 1)
In patients WS1, WS2, and WS3, epileptic seizures were controlled within 6 months after the appearance of their initial symptoms. In LS1, it took 3 years for seizures to cease. In spite of this difference, the scores of all four patients decreased with age, and became 0, although it took 2 to 4 years after epileptic seizures disappeared.
The intractable group (Fig. 2)
No patients in this group showed a zero score of sleep-wakefulness rhythm during the observation period. Moreover, the scores increased in five patients (WS4, WS6, LS3, LS4, and LS6). These increases were followed by a deterioration of the epileptic seizures.
Discussion
The current study has several methodological limitations. First of all, the method we used - the sleep log - is not a direct method of assessing sleep habits. However, at least in young children, the sleep measures reported by guardians showed significant correlations with objective sleep measures determined by actigraphy (Sadeh, 1996). In addition, a sleep log allows us to obtain longitudinal data on sleep habits. Utilizing this advantage, we used sleep logs to evaluate sleep habits of our patients for several years, even though it was an indirect method. Secondly, the effects of the various anticonvulsants could not be neglected. However, by using non WS and non LS epileptic patients who also received anticonvulsants as controls, we managed to minimize the effects of anticonvulsants in our comparisons of the sleep-wakefulness rhythms, although the number of anticonvulsants was not controlled between the subject patients and the controls.
In general, sleep deprivation facilitates both epileptiform abnormalities (Bennet, 1963) and seizures (Bennet, 1963; Gunderson, et al. 1973). Seizures are well known to impact sleep (Méndez and Radtke 2001), and in child patients with epilepsies, various sleep abnormalities have been demonstrated (Janz, 1974; Besset, 1982; Kellaway, 1985; Zaivalla, 1989). Konishi, et al. (1995) tried add-on therapy with flunitrazepam in five child patients with marked irregularities of sleep-wakefulness rhythm and intractable seizures. They obtained corrections of their sleep-wakefulness disturbances, decreases in seizure frequency, and improvements in the quality of life within a year in four of the five patients.
The sleep-wakefulness rhythms of our benign group became indistinguishable with those of well-controlled non-WS or non-LS epileptic patients within a few years, while the sleep-wakefulness rhythms of our intractable group showed marked differences from that of well-controlled non-WS or non-LS epileptic patients. In addition, in contrast to Konishi, et al. (1995), it took 2 to 4 years after epileptic seizures disappeared until the scores for the sleep-wakefulness rhythm became zero in our benign group. The current results suggested that disturbances of the sleep-wakefulness rhythm are more severe in patients with WS and LS than in child patients with other types of epilepsy. The common neuronal disturbances that WS and LS are presumed to share (Ohtahara, 1984) are postulated to be implicated in systems regulating the sleep-wakefulness rhythm.
We previously reported disturbances of phasic chin muscle activity in WS patients (Kohyama, et al. 1996) and LS (Iwakawa, et al. 1984). Activities of the noradrenergic (Mileykovskiy, et al. 2000) and serotonergic (Trulson and Jacobs, 1981) neurons in the brainstem are considered to be involved in the occurrence of phasic muscle activity during sleep. These monoaminergic systems might be impaired in patients with WS and LS. We also reported delayed maturation of sleep-wakefulness rhythm in a boy whose mother was administered alpha-methyldopa during pregnancy (Shimohira, et al. 1986). During infancy, he showed delayed onset of nocturnal sleep and long daytime sleep duration, which was corrected by one year of age. Since maternally administered alpha-methyldopa could enter a placenta and act as a psuedotransmitter, the sleep disturbances of this boy were suggested to be the result of interference by monoaminergic metabolisms in his developing brain (Shimohira, et al. 1986). Disturbances of monoaminergic systems are likely to be involved not only in the occurrence of WS and LS but also in the impairment of the sleep-wakefulness rhythm.
The suprachiasmatic nuclei of the anterior hypothalamus functions as a circadian clock, and plays an important role in the regulation of various inner biological rhythms. The suprachiasmatic nucleus sends both direct and indirect projections to the dorsomedial hypothalamus (Chou, et al. 2002). In turn, the dorsomedial hypothalamus sends a heavy output to the ventrolateral preoptic nucleus (Chou, et al. 2002). The ventrolateral preoptic nucleus and monoaminergic nuclei in the tuberomammillary nucleus, raphe nuclei, and the locus coeruleus are connected reciprocally. Thus, the biological clock and brainstem monoaminergic systems are closely interconnected, although indirectly (Chou, et al. 2002). A disturbance in this interconnectivity between the hypothalamus and the brainstem, as well as in the brainstem monoaminergic systems themselves, could affect the biological clock. Although only in the WS cases, we proposed that functional rostral brainstem impairment was responsible for the occurrence of the disease (Kohyama, et al. 1996).
Thus, it is speculated that disturbances of brainstem monoaminergic systems or the impairment of reciprocal interconnections between the hypothalamus and the brainstem monoaminergic systems are responsible for the disturbances of the sleep-wakefulness rhythm in patients with WS and LS.
References
Bennet, D.R. Sleep deprivation and major motor convulsions. Neurology, 1963, 13: 953-958.
Besset, A. Influence of generalized seizures on sleep organization. In: M.B. Sterman, M.N. Shouse, P. Passouant (Eds) Sleep and Epilepsy. New York, Academic Press, 1982: 109-118.
Campos-Castello, J. Therapeutic strategy in severe encephalopathies. Rev. Neurol., 2001, 32: 860-866.
Chou, T.C., Bjorkum, A.A., Gaus, S.E., Lu, J., Scammell, T.E., Saper, C.B. Afferents to the ventrolateral preoptic nucleus. J. Neurosci., 2002, 22: 977-990.
Gunderson, C.H., Dunne, P.B., Feyer, T.L. Sleep deprivation seizures. Neurology, 1973, 23: 678-686.
Iwakawa, Y., Ogiso, M., Toyoda, M., Hosaka, S., Niwa, T., Dan, T., Segawa, M. Body movements during sleep in Lennox syndrome. No To Shinkei, 1984, 36: 267-273, (in Japanese).
Jan, J.E. and O'Donnell, M.E. Use of melatonin in the treatment of pediatric sleep disorders. J. Pineal. Res., 1996, 21: 193-199.
Janz, D. Epilepsy and sleeping-waking cycle. In: P.J. Vinken, G.W. Bruyn (Eds) The Epilepsies. Amsterdam, Elsenier, 1974: 457-490.
Kellaway, P. Sleep and epilepsy. Epilepsia, 1985, 26 (suppl 1): S15-S30.
Kohyama, J., Ohsawa, Y., Shimohira, M., Iwakawa, Y. Phasic motor activity reduction occurring with horizontal rapid eye movements during REM sleep is disturbed in infantile spasms. J. Neurol. Sci., 1996, 138: 82-87.
Konishi, T., Masuko, K., Naganuma, Y., Hongou, K., Yagi, S. Flunitrazepam for sleep disturbance in children with intractable epilepsy. Brain Dev., 1995, 17: 69-72.
Méndez, M. and Radtke, R.A. Interactions between sleep and epilepsy. J. Clin. Neurophysiol., 2001, 18: 106-127.
Mileykovskiy, B.Y., Kiyashchenko, L.I., Kodama, T., Lai, Y.Y., Siegel, J.M. Activation of pontine and medullary motor inhibitory regions reduces discharge in neurons located in the locus coeruleus and the anatomical equivalent of the midbrain locomotor region. J. Neurosci., 2000, 20: 8551-8558.
Ohtahara, S. Seizure disorders in infancy and childhood. Brain Dev., 1984, 6: 509-519.
Okawa, M. and Sasaki, H. Sleep disorders in mentally retarded and brain-impaired children. In: C. Guilleminault (Ed) Sleep and Its Disorders in Children. New York, Raven Press, 1987: 269-290.
Sadeh, A. Evaluating night wakings in sleep-disturbed infants: a methodological study of parental reports and actigraphy. Sleep, 1996, 19: 757-762.
Shimohira, M., Kohyama, J., Kawano, Y., Suzuki, H., Ogiso, M., Iwakawa, Y. Effect of alpha-methyldopa administration during pregnancy on the development of a child's sleep. Brain Dev., 1986, 8: 416-423.
Trulson, M.E. and Jacobs, B.L. Activity of serotonin-containing neurons in freely moving cats. In: B.L. Jacobs and A. Gelperin (Eds) Serotonin, Neurotransmission and Behavior. Cambridge, MIT Press, 1981: 339-365.
Zaivalla, Z. Sleep abnormalities in children with epilepsy. Electroencephalogr. Clin. Neurophysiol., 1989, 72: 29.
Tables
| Patient |
Sex |
Diagnosis |
Age of seizure onset |
History |
| Benign group |
| WS 1 |
F |
West syn. |
5m |
- |
| WS 2 |
M |
West syn. |
6m |
Neonatal asphixia |
| WS 3 |
M |
West syn. |
1y4m |
- |
| LS 1 |
M |
Lennox syn. |
3y3m |
- |
| Intractable group |
| WS 4 |
F |
West syn. |
1m |
Neonatal asphixia |
| WS 5 |
M |
West syn. |
1y |
- |
| WS 6 |
F |
West syn. |
8m |
Microcephalus |
| LS 2 |
M |
Lennox syn. |
3y11m |
- |
| LS 3 |
M |
Lennox syn. |
1d |
Neonatal asphixia |
| LS 4 |
F |
Lennox syn. |
8m |
Tuberous sclerosis |
| LS 5 |
M |
Lennox syn. |
5m |
Tuberous sclerosis |
| LS 6 |
M |
Lennox syn. |
7m |
Prurent meningitis |
Table 1. Patient profiles
Figures
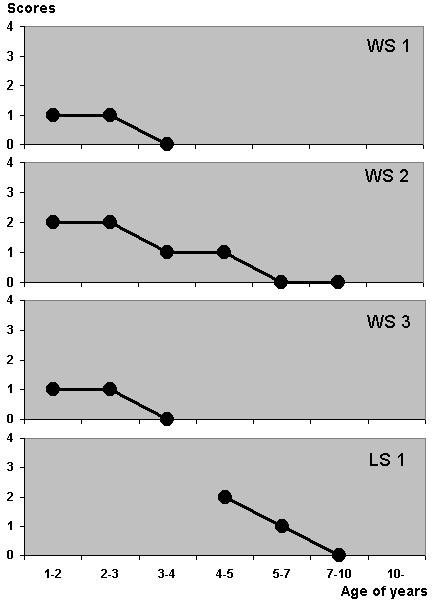
Figure 1. Chronological changes of sleep-wakefulness rhythm scores in the benign group. Scores of all four patients decreased to zero with age.
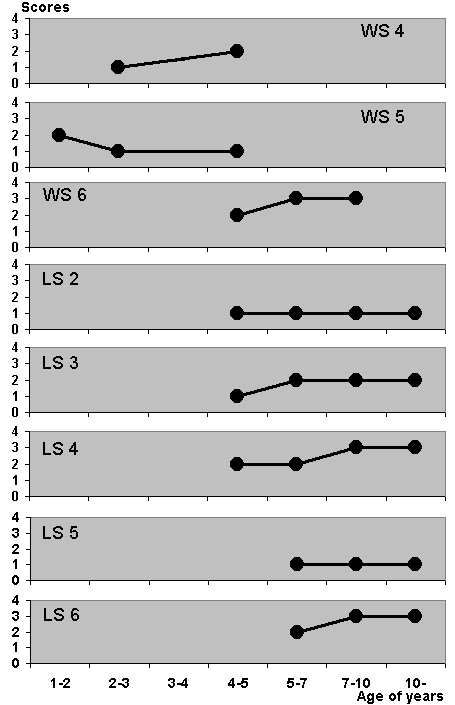
Figure 2. Chronological changes of the sleep-wakefulness rhythm scores in the intractable group. No patients in this group show a zero score, with the score increasing in five patients (WS4, WS6, LS3, LS4, LS6).
Correspondence: Kohyama Jun, PhD.,
Division of Human Ontogeny and Childhood Development,
Graduate School, Tokio Medical and Dental University,
1-5-45, Yushima, Tokio, 113-8519, Japan.
E-mail: jkohyama.ped@tmd.ac.jp

