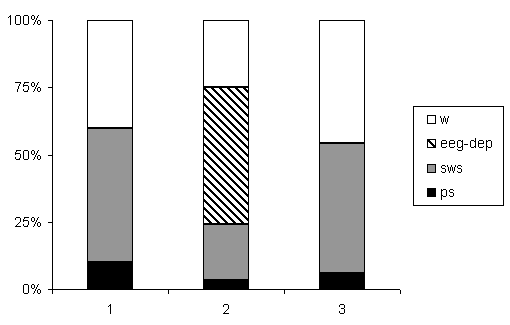1. Changes in electrical activity of cortical areas at administration of narcotic and half-narcotic doses of E. At IP administration of narcotic dose of E (4,5g/kg) the corticogram was considerably changed. Fig.1 shows a record of the cortical areas until injection (A), 15 min. after injection (B) and 2 h later (C). As it is demonstrated, electrical activity of the cortex undergoes important changes. The amplitude was suppressed to EEG-depression. At the same time complete immobilization with muscle relaxation and spinal reflexes inhibition were observed.
Two hours later motor reflexes were partially restored. Synchronized low amplitude rhythm (in ratio 4/sec) was registered. It is very important to note that during such activity the SW-C had not been revealed. The motor activity was restored after 3-4 hours while the SW-C was not.
Half-narcotic dose (2.25 g/kg) of E evoked less EEG-effect and its duration was significantly shorter.
2. Changes of ratio of phases in the SW-C at narcotic and half-narcotic doses of E. Administration of narcotic dose of E evoked significant changes in the SW-C. As mentioned above during EEG-depress in the SW-C had not been generated.
Percentage ratio of various phases in the successive SW-C after E administration is shown in Fig.2. A single injection of E (4,5 g/kg) caused significant decrease of all phases of the SW-C. Wakefulness decreased from 40% to 29%, SWS reduced from 53% to 29%, PS was suppressed from 7% to 2%. EEG-depression occupied 39% of the total experimental time. As it was mentioned above, polygraph recording of the cycle was continued on the second day of E treatment. The total duration of wakefulness was longer as compared to the background; the total length of SWS and PS consequently was suppressed.
In order to assertion the cause of disturbance of the normal of the SW-C under E administration, a detailed analysis of the cyclograms was carried out. An analysis of the calculation of data had shown that average duration of phases in the cycle as compared to the background was changed. This was connected to duration of wakefulness and SWS, the average length of PS was not changed (Fig.3), but the frequency of PS significantly decreased (Fig.6, A1). The latency of SWS increased (Fig.4) and the frequency of arousal also enhanced (Fig.5, A1) and thus the structure of the SW-C became fragmentary.
Half-narcotic dose of E at minor influence on the EEG-activity evoked less disturbance of the structure of the SW-C. The cycle developed with all its phases. But the total duration of SWS as well as PS was shorter and the total length of wakefulness was rather longer as compared to the background (Fig. 3, A2). At the same time average duration wakefulness and SWS was shorter, the frequency of PS was lower as compared to the base-line, but higher than during at E narcotic dose administration (Fig.6, A2) the latency of SWS before PS manifestation was enhanced (Fig.3, A2). The frequency of awakenings was higher (Fig.5, A2) and so the structure of SW-C was fragmented. Therefore the disturbance of the SW-C at the treatment with narcotic dose of E was development due to EEG-depression.
Blum, K., Futtermans, G., Wallace, J.E., Schwetener H.A. Naloxone-induced inhibition of ethanol dependence in mice. Nature, 1977, 265: 49-51.
Borbely, A.A. A two proceses model of sleep regulation. 1: Physiological basis and out line. Hum. Neurobiol., 1982, 1: 195-204.
Burov, Yu., Viglinskaia, Y.V. The influence of psychotropic agents on sleep disturbances during alcohol deprivation in rats. Bull. Exp. Biol. Med., 1981, 6: 689-691.
Canches-Perez, A.M., McMahon, T., Mehnert, K., Kelley, S.P., Hodge, C.W., Messing, R.O. Increased acute responses to ethanol and sensitivity of GABA-A receptors to ethanol and benzodiazepines in PKC epsilon mutant mice. Alcoholism. Clin. and Exp. Res., 1999, 23, 5: 19A.
Gillin, J.C., Kripke, D.F., Risc, S.C., Schuckit, M. Slow waves sleep. Relationship to primary alcoholism and to sleep deprivation in depression. In: A. Wauquer et al., (Ed.) Slow Wave Sleep: Physiological and Functional Aspects, Raven Press, LTD., N-Y. 1989: 141-154.
Goodwin, F.L.W., Campi, M., Babinska, I., Amit, Z. The effects of naltrexone on intake of ethanol and floured solution in rats. Alcoholism. Clin. and Exp. Res., 1999, 23, 5: 16A.
Hanania, T., Negri, C.A., Dundwiddie, T.V., Zahniser N.R. Modulation of NMDA receptor function by NMDA receptor antagoniasts and ethanol in bred of long sleep and short sleep mice: behavior and electrophysiology. Alcoholism. Clin. and Exp. Res., 1999, 23, 5: 9A.
Hobson, J.A., Pace-Schott, E.E., Stickgold, R., Kahn, D. To dream or not to dream? Relevant data from new neuroimaging and electrophysiological studies. Current Opinion in Neurobiology, 1998, 8: 239-244.
Jouvet, M. The role of monoamines and acetylcholine containing neurons in the regulation of sleep-waking cycle. Ergbn. Physiol., 1972, 64: 166.
Maurel, S., De Vry, J., De Beun, R., Schrieber, R. 5-HT-A and 5-HT C/5-HT 1B receptors are differently involved in alcohol preference and consummatory behavior in cAA rats. Pharmacology, Biochemistry and Behavior, 1999, 62, 1: 89-96.
Moruzzi G. The sleep waking cycle. Ergebn. Physiol., 1972, 64, 64.
Oniani, T.N. Integrative Function of the Limbic System. Tbilisi, Metsniereba, 1980, 5-27, (in Russian).
Snyder, S., Karacan, J., More, C., Sleep patterns of sober chronic alcoholics. Sleep Res., 1984, 13: 182.
Tobler, I., Fraken, P., Trachel, L., Borbely A.A. Models of seep regulation in mammals. J. Sleep Res., 1992, 1: 125-127.
Vein, A.M., Vlasov, M.A., Dllakian, J.G., Eligulashvili, T.S., Kulikovski, V.V., Levin, Y.Y., Sidorov A.A. The adaptive role of delta sleeps. Fiziologia Cheloveka, 1985, II, 2: 252-257, (Russian).
Volpiselli J.R. Uncontrollable events and alcohol drinking. British Journal of Addiction, 1987, 82: 381-392.
Yules, R.B., Lippman, M.E., Freedman, D.X. Alcohol administration prior to sleep. The effect of EEG sleeps stages. Arch. Gen. Psychiatry,, 1967, 19: 94.
Zarcone, V.P., Scheier, L., Mitchell, G., Oremberg, E., Barchas, J. Sleep variables, cyclic AMP and biogenoc amine metabolites avter one day ethanol injection. J. Stud. Alcohol., 1980, 41: 318-324.
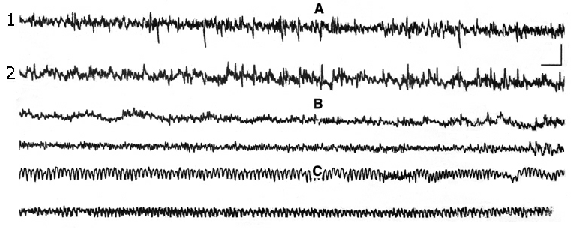
Figure 1. Electroencephalograph changes of different areas of new cortex at narcotic dose of ethanol.
1. SM-cortex, 2. occipital area (hippocampal projection). A - background, B - after 15 min of ethanol administration, C - after 2 hours of injection. Calibration - 1 sec, 100mV.
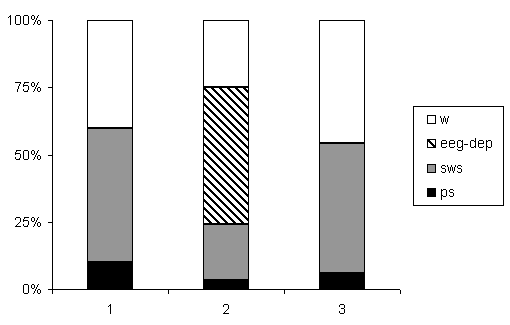
Figure 2. Changes of ratio of phases in the SW-C at narcotic and moderate doses of ethanol.
B - background, A1 - at narcotic doses (4.5 g/kg), A2 - at moderate dose (2.25 g/kg).
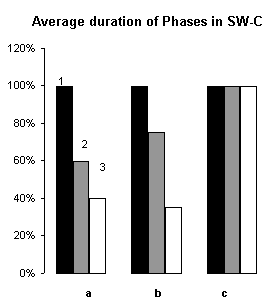
Figure 3. Changes of average duration of phases in SW-C and latency of SWS.
I. A - wakefulness, B - slow wave sleep, C - paradoxical sleep; 1 - background, 2 - after narcotic dose administration, 3 - at moderate dose.
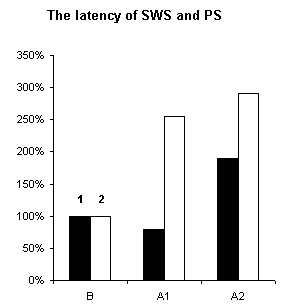
Figure 4. Changes of latency of SWS and PS during of ethanol administration.
B - background, A1 - after narcotic dose administration, A2 - at moderate dose. 1 - SWS, 2 - PS
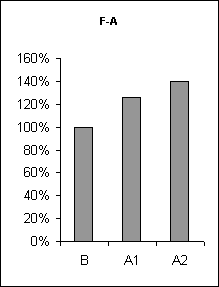
Figure 5. The frequency of awakenings at ethanol administration.
B - background, A1 - at narcotic dose, A2 - at moderate dose.
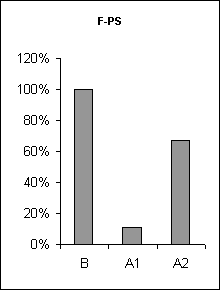
Figure 6. The frequency of paradoxical sleep onset at ethanol administration.
B - background, A1 - after narcotic dose administration, A2 - at moderate dose.