Neurobiology of Sleep-Wakefulness Cycle 1(2): 52 - 81, 2001
THE ROLE OF THE SEPTO-HIPPOCAMPAL SYSTEM AND PARADOXICAL PHASE OF SLEEP IN THE REGULATION OF LEARNING AND MEMORY(See remark)
Department of Neurobiology of Sleep-Wakefulness Cycle, I.S. Beritashvili Institute of Physiology, Georgian Academy of Sciences, Tbilisi
Accepted in revised form 18 December 2001; recieved 15 August 2001.
Summary
In cats with chronically implanted electrodes in different brain structures study was made of the effect of postconvulsive depression of the hippocampal theta rhythm before and after learning sessions on the elaboration and extinction of instrumental alimentary reflexes based on sound discrimination; the effect of paradoxical sleep deprivation (PSD) by the method of non-emotional awakening, which prevents the development of the hippocampal theta rhythm on the elaboration and extinction of instrumental alimentary reflexes based on sound discrimination; the effect of lesion of various septal areas on (a) general behavior, (b) the sleep-wakefulness cycle, (c) the elaboration and extinction of these reflexes and (d) the conditioned delayed responses; the effect of functional elimination of the hippocampus by way of induction of local epileptiform discharges in it on the elaboration and extinction of instrumental conditioned alimentary reflexes and on conditioned delayed responses. The data were statistically processed.
Analysis of our experimental results allows to come to the following conclusion: 1. Depression of the hippocampal theta rhythm by way of repeated epileptiform discharges both prior to and following learning sessions leads to a considerable retardation of elaboration of instrumental alimentary reflexes in the initial sessions but does not prevent subsequent elaboration of discrimination of conditioned signals. 2. Depression of the hippocampal theta rhythm by way of inducing in it repeated epileptiform discharges before and after learning sessions has no considerable effect on the extinction of conditioned instrumental alimentary reflexes. 3. PSD in the intersession period and thus prevention of the development of the hippocampal theta rhythm without any accessory changes in the functional state of the brain has no significant effect on the elaboration of instrumental alimentary reflexes based on sound discrimination. 4. Lesion of the medial septum, causing a complete abolition of the hippocampal theta rhythm, has no tangible effect either on the structure of the sleep-wakefulness cycle or on the cat's general behavior: the signs of the so-called "septal syndrome" are lacking. The rate of elaboration of instrumental alimentary reflexes based on sound discrimination and extinction of these reflexes remains unchanged. Yet, lesion of the medial septum results in the disturbance of delayed responses to conditioned signals. 5. Massive lesion of the septum, when, apart from the medial area, the lateral one is also involved, considerably alters the structure of the sleep-wakefulness cycle (wakefulness becoming longer and PS shorter) and the character of general behavior. The cats are rendered more agitated, aggressive, diverted and hyperphagic. Motor activity also augments significantly. All these changes are the components of the "septal syndrome". Elaboration of instrumental alimentary reflexes in such animals is hindered, especially affected is the elaboration of discrimination of conditioned sounds because of the development of perseverative movements. The process of extinction of instrumental alimentary reflexes is sharply retarded and the delayed responses to conditioned signals are completely disturbed. 6. Functional elimination of the hippocampus by way of inducing in it epileptiform discharges in various intervals (1 min, 25 sec, immediately) after each pairing of a conditioned signal with food reinforcement facilitates the elaboration of instrumental alimentary reflexes based on sound discrimination. Such epileptiform discharges accelerate also the extinction of conditioned alimentary reflexes when they are induced immediately after the animal approaches an empty feeder, and has no effect if they are induced l min after approach. 7. Epileptiform discharges in the hippocampus induced immediately or 2-5 min after the onset of this or another conditioned signal disturb the delayed responses and have no effect if they are induced at long intervals.
The role of the septo-hippocampal system in the regulation of learning and memory is discussed on the basis of the foregoing. The view is advanced and substantiated according to which the descending influence of the hippocampus via the lateral septum on the activating and motivating structures of the mesodiencephalon is more important in the regulation of these processes than is the ascending influence of the mesodiencephalon via the medial septum, as a result of which the theta rhythm is formed at the hippocampal level.
Moreover, it has been revealed that the septo-hippocampal system plays a crucial role in the regulation of short-term operative memory responsible for delayed conditioned reactions. What is more, in the regulation of memory of this type both the input (the medial septum) and the output (the lateral septum) of the hippocampus are equally important.
Key Words: Hippocampus, Septum, Theta rhythm, Learning, Memory
Introduction
The role of the hippocampus in the regulation of recent memory was suggested in the clinical studies of Bekhterev (1900) and Penfield and Milner (1958). Experimental analysis of their observations yielded contradictory data and the differences of opinion remaining unresolved to the present day (Douglas 1967; Hostetter 1968; Milner 1968; Mering et al. 1972; Vinogradova 1975; Oniani 1980). In spite of this, over the last two decades a large number of publications have appeared, supporting the important role of the hippocampus in the regulation of learning and memory. In considering this question particular attention is devoted to the dynamics of the hippocampal electrical activity. The initial position taken is that the dynamics of the hippocampal theta rhythm reflects the different levels of functioning of the septo-hippocampal system, as it is known that the pacemaker for the hippocampal theta rhythm is localized in the septum (Petsche and Stumpf 1960; Petsche et al. 1962) and generation of this activity occurs in the pyramidal cell layers of the hippocampus (Green et al. 1960; Green and Petsche 1961).
As early as in l938 Jung and Kornmuller (1938) noticed that in mammals the transition from quiet wakefulness or sleep to active wakefulness is paralleled by a hypersynchronization of slow potentials in the hippocampus. This phenomenon, however, was not commonly recognized until after the appearance of the classical paper of Green and Arduini (1954), who demonstrated that integrity of the hypothalamus and septum is necessary for the occurrence of the hippocampal theta rhythm during arousal. Lesion of these structures leads to the disappearance of the theta rhythm in the hippocampus.
All-round analysis of the hippocampal theta rhythm has since become one of the most intriguing problems of neurophysiology, leading to the emergence of several theories dealing with the possible significance of this bioelectric phenomenon as an indicator of one or another function inherent in the hippocampus. Comprehensive surveys of these theories have been presented by a number of authors (Douglas 1967; Vinogradova 1975; Isaacson 1976; Bennet 1977; Oniani 1980).
The most popular theory at present is the one according to which the dynamics of the hippocampal theta rhythm reflects different steps of the organization of learning and memory. Grastyan and co-workers (Grastyan et al. 1959) and later Pickenheim and Klingberg (1967) made a detailed study of the dynamics of the hippocampal theta rhythm during the elaboration of conditioned reflexes. They found that in response to a conditioned signal in the initial phases of learning the hippocampal theta rhythm develops well but, as the conditioned reflex consolidates, it ceases to occur. The same was observed by Morrel (1961) in rabbits in respect to the dynamics of synchronized activity of the brain. From these data it is clear that the theta rhythm must be related to a nonspecific activation of the brain. Subsequently, however, Adey et al. (Adey 1961, 1962, 1966) asserted that the theta activity of the hippocampus is related to the registration, storage and retrieval of information. If hippocampal theta rhythm is blocked both learning and retrieval of memory traces become impossible.
According to McGough (Landfield et al. 1972; McGaugh 1966, 1972, 1973) and Landfield and co-workers (Landfield et al. 1972) the best storage of learning is shown by animals in which the theta rhythm is well pronounced immediately after the learning session. Conversely, those having ill-pronounced theta activity in the post-training period showed the worst storage of the learned task. In the opinion of McGaugh (1972), "theta activity may be a correlate of the process underlying the formation or consolidation of long-lasting or stable traces of memory". Vinogradova (Vinogradova 1975; Vinogradova and Braznik 1978) claims that "the range of theta rhythm is the measure of the dynamic range of optimal information input and processing", while according to Ott and co-workers both electrical (Wetzel et al. 1977) and chemical (Ott 1979) stimulation of the brain results in an increase of the hippocampal regular synchronous activity, facilitating thereby the process of learning.
The idea of the significance of the theta rhythm in the organization of memory especially attracts those workers who study the role of paradoxical sleep (PS) in the process of learning (Bloch 1970; Fishbein 1970; Fishbein et al. 1971). In their opinion, PS plays an important role in the processing of information, and since the hippocampal theta rhythm is particularly pronounced in this phase, it must be related to the process of consolidation of memory traces.
Papers have appeared recently (Bennett 1977; Oniani 1979) in which the specific significance of the development of the hippocampal theta rhythm for the organization of learning and memory is questioned. At the same time it is suggested that the generation of the hippocampal theta rhythm is related to a nonspecific activation of the brain, which in turn is necessary for the realization of all steps of learning and memory organization (Hebb 1949). It has been suggested that neither development of the hippocampal theta rhythm nor the hippocampus per se is necessary for the memory trace consolidation (Hirano 1965; Anschel C. and Anschel S. 1969; Oniani and Vartanova 1980). On the basis of experimental data obtained in our laboratory over the last ten years (Oniani 1980) we concluded that the hippocampal theta rhythm is not a specific correlate of some behavior or of some step of memory organization during learning, but rather a nonspecific correlate of emotional tension due to the activation of mesodiencephalic structures playing a role in motivated functions.
As was mentioned above, the role of the hippocampus in memory was shown first in clinical works with patients having lesions in this structure (Penfield and Milner 1958; Milner 1968). These studies revealed that hippocampal lesions impair the memory of recent events, but not that of older ones, which indicates that the hippocampus somehow contributes to the consolidation of memory traces and to the conversion of recent into long-term memory.
Later on, there appeared numerous studies dealing with the participation of the hippocampus in the processes of consolidation of memory traces. In the first place, mention should be made of studies in which the amnesic action of electroconvulsive shock is attributed to the development at this time of convulsive activity in the hippocampus (Hostetter 1968; Barcik 1970; McGaugh 1973). This is allegedly evidenced in Hostetter's (1968) data showing that electroconvulsive shock in hippocampectomized rats causes considerably less retrograde amnesia than in intact animals. However, in a more recent work by Carl (1979) this observation was not confirmed.
A more detailed specification of the structures involved in the mechanisms of memory was made by means of local electrical stimulation of discrete brain areas. If electrical stimulation of the hippocampus, producing epileptiform discharges, is applied after the pairing of conditioned and unconditioned stimuli, it has an amnesic effect. The longer the interval between the trial and stimulation of the hippocampus the less pronounced is this effect, or vice versa (Kesner and Doty 1968; McDonough and Kesner 1971; Vardaris and Schwartz 1971; Shinkman and Kaufman 1972; Wetzel et al. 1977). Other investigators observed facilitation of learning when electrical stimulation of the hippocampus did not induce epileptiform discharges (Destrade and Cardo 1974; Soumireu-Mourat et al. 1975; Gralewicz 1976). Stein and Chorover (1968) found that stimulation of the hippocampus, applied after each trial of learning, improved task performance in rats, even though the stimulation did induce characteristic epileptiform discharges. They conclude that the reason for this is a facilitation of memory trace consolidation. On the other hand, Anschel C. and Anschel S. (1969) as well as Hirano (1965) assume that the hippocampus does not participate in the consolidation of memory traces. From the foregoing it follows that in order to solve the problems of participation of the hippocampus in memory organization and of the significance of the dynamics of the hippocampal theta rhythm reflecting different levels of functioning of the septo-hippocampal system for memory and consolidation, further studies are needed with various approaches and methods. To this end, we have studied: 1) The effect of postconvulsive depression of the hippocampal theta rhythm on the elaboration and extinction of instrumental alimentary reflexes, 2) The effect of abolishment of the hippocampal theta rhythm by PS deprivation (PSD) on the elaboration of instrumental alimentary reflexes, 3) The effect of the abolishment of this rhythm by means of electrocoagulation of the septal input on the elaboration and extinction of instrumental conditioned alimentary reflexes and on delayed reactions, and 4) The effect of functional elimination of the hippocampus by induction of local EEG epileptiform discharges on the elaboration and extinction of instrumental alimentary reflexes and on delayed reactions.
Methods
Animals. Experiments were carried out on 35 mature cats of both sexes. Before the commencement of learning sessions and stimulation the animals were adapted to the experimental chamber and observation was made of their general behavior. The amount of their daily food intake was also measured. Later the cats were selected for the experiments which had been easily adapted to the experimental design, had good appetite and were not distinguished by aggressiveness or apprehensiveness, i.e. were steady.
Implantation of electrodes. Metallic electrodes were stereotaxically implanted to stimulate the brain structures electrically and to record the focal electrical activity from the neocortex and hippocampus. Surgery was made under Nembutal anesthesia (35 mg/kg). The coordinates of the cat's brain atlas of Jasper and Ajmone-Marsan were used (1954).
Procedure of elaboration of instrumental alimentary reflexes. Elaboration of instrumental alimentary reflexes was effected in a special experimental chamber (Fig. 1) consisting of two compartments. The rear part of the chamber with the area of 0.3 m2 served as the starting place where the animals were kept in the intertrial period. In the front compartment with the area of 1 m2 outside at the front side-walls feeders were placed from which the animal received food reinforcement by raising the suspended door. Before starting the elaboration of instrumental food-motor conditioned reflexes to two feeders, the animals had been, for several days, adapted to the experimental chamber. Within this time they learned to open the suspended door with the forepaws and procure a piece of meat from the feeders fastened outside to the front edge of the chamber side-walls. Thereafter conditioned food-motor reflexes to sound discrimination were elaborated namely to the two feeders. Tone of 500 Hz served as a conditioned signal for one feeder, and clicks for the other. In response to the former, the animal was expected to go to the right, in response to the latter, to the left. The source of sound was placed on the top of the front wall at the very ceiling of experimental chamber. 5 sec after the onset of one or the other signal the door of the starting section was opened and the cat was allowed to perform the food-procuring behavior.
After reaching 100 % discrimination of the conditioned signals delayed reactions were measured, and then we proceeded to the extinction of conditioned reflexes. In 10 cats, which had not been subjected to electrical stimulation of the hippocampus and lesion of the septum, the rate of elaboration and extinction of instrumental alimentary reflexes as well as the duration of delayed reactions to two feeders in response to the conditioned signals were measured. The mean data obtained in these cats served as a standard for comparison with those obtained in the experimental ones.
Brain stimulation. The dorsal hippocampus was stimulated by rectangular pulses from a generator of high-frequency output. The stimulation parameters for inducing of hippocampal epileptiform discharges were selected for each cat. The stimulus frequency was usually 200-300 per sec, duration 0.1 msec, and the intensity varied within a considerable range (from 3 to 6 V). The course of combination of the hippocampal electrical stimulation with the sessions and trials of learning will be described in the relevant sections when estimating the experimental data.
Septal lesion. The septum was lesioned by electrocoagulation. For this purpose, on each side of the brain in the medial septum bipolar electrodes were implanted with 2 mm difference in length. Direct current of the intensity of 30-50 mA was passed during 20-40 sec, first through one electrode and then through the other. The extent of septal lesion varied depending on the intensity of direct current and duration of its passage.
Polygraphic recording. Recording of electrical activity in the neocortex and hippocampus as well as of evoked epileptiform discharges was effected on an ink-writing electroencephalograph of the firm "San'ei". In order to identify different phases of the sleep-wakefulness cycle, apart from the electrical activity of the above structures, recording was made also of the cervical muscles and rapid eye movements.
Histology. To specify the localization of deep electrodes after the termination of experiments direct current (20 mA for 20 sec) was passed through them and fixation of the brain was made by perfusion of neutral formalin through the carotid artery. The above surgery was made under Nembutal anesthesia (35 mg/kg). For further fixation the brain was removed from the skull and was placed for some days again in neutral formalin. Then the exact localization of electrodes and the septal lesion extent were determined in frontal serial sections.
Data processing. The data were statistically treated. Mean values were calculated, their standard deviations and the validity of difference in the above mean values were checked by Student's t-test.
Results
Influence of postconvulsive depression of the hippocampal theta rhythm on learning and memory.
These series of experiments were carried out on three groups of animals. The first group involved 10 control cats in which the rate of elaboration and extinction of conditioned reflexes, as well as delayed reactions, were measured.
The data obtained in this group served as a reference for the data obtained in all experimental groups described in this paper. The influence of preliminary depression of the hippocampal theta rhythm on the elaboration and extinction of conditioned reflexes was studied in 5 cats. For this purpose, before the beginning of elaboration and extinction, repeated daily electrical stimulation was applied to the hippocampus, inducing local epileptiform discharges. This procedure lasted for 2 hours with 2-3 min intervals. In order to elicit more or less local epileptiform discharges in the hippocampus electrical stimulation was applied to its dorsal part. Chronically implanted stimulating metallic electrodes had been localized in that layer from which a pronounced theta rhythm was to be recorded at active wakefulness and against the background of paradoxical phase of sleep. Usually at the threshold high-frequency (100-200 per sec) electrical stimulation development of the phenomenon of desynchronization without elicitation of discharges was observed both in the contralateral hippocampus and on the level of neocortex. With an increase of stimulation intensity the epileptiform discharges start to appear in the form of aftereffects, while at further increase, the epileptiform discharges localized in the hippocampus might start shortly after the application of electrical stimulation, i.e. against its background. In our experiments both parameters of stimulation were used. A typical picture of development of more or less local epileptiform discharges in the hippocampus is presented in Fig. 2. As is seen, with the given parameters of electrical stimulation, the local epileptiform discharges develop in the hippocampus in the form of after-effects and they last from 15 to 20 sec. During electrical stimulation, before development of epileptiform discharges, one can observe the phenomenon of desynchronization (Fig. 2A) both in the neocortex and the hippocampus, but on the face of epileptiform discharges there is an acute enhancement of b1 and b2 rhythms in the hippocampus, while in the sensorimotor area of the neocortex there is a preferential enhancement of d and q rhythms because of the tendency of this structure to synchronization. Fig. 2C shows that the local epileptiform discharges elicited by electrical stimulation of the dorsal hippocampus spread throughout its ventral area i.e. embrace bilaterally all the areas of the hippocampus. After the cessation of epileptiform discharges one can see an apparent depression of all hippocampal rhythms (Fig. 3A), meanwhile the rhythms in the sensorimotor area of the neocortex do not considerably alter (Fig. 3B). This indicates that in these experiments a more or less pronounced functional elimination occurs namely in the hippocampus and not in the structures of neocortex.
Experimentally elicited local epileptiform discharges in the hippocampus seem to exert an especially depressing influence on the functional state of the septo-hippocampal system. This is primarily indicated by the occurrence of post˝onvulsive potent and prolonged suppression of the hippocampal theta rhythm. The latter continues even after the retarded restoration of the sleep-wakefulness cycle. As an illustration of the above-said serves Fig. 4 where comparison is made of the expression of all major rhythms in the dorsal hippocampus on the background of paradoxical sleep before elicitation of repeated local epileptiform discharges with expression of the same rhythms in the first paradoxical phase following recovery of the sleep-wakefulness cycle. It should be noted that following 15 short-lasting elicited epileptiform discharges the sleep-wakefulness cycle was restored only after three hours and was expressed in the slow wave sleep onset, and the first PS appeared only in four hours after the last elicited epileptiform discharge.
In the context of participation of the hippocampus in learning and consolidation of memory traces it is interesting to mention also the fact that its electrical stimulation in the above-indicated experiments did not lead to the activation of already elaborated food-motor conditioned reflex, i.e. retrieval of memory traces. As is known, activation of already elaborated food-motor conditioned reflexes occurs at the stimulation of the brain structures such as the lateral hypothalamus and the baso-lateral part of the amygdala (Delgado et al. 1954; Andersson and Wyrwicka 1957; Grossman 1962; Oniani et al. 1976; Oniani 1979). At the same time, in response to electrical stimulation of the mentioned structures, the EEG desynchronization in the neocortex is paralleled with the generation of pronounced theta rhythm in the hippocampus. In contrast to this, high-frequency threshold electrical stimulation of the dorsal hippocampus brings about the phenomenon of EEG desynchronization on the level of neocortex, as well as in the hippocampus without clear-cut development of theta rhythm in the latter (Fig. 2A). At the moderate suprathreshold stimulation, epileptiform discharges are generated within hippocampus, again with EEG desynchronization on the level of neocortex (Fig. 2C). While after cessation of the elicited epileptiform discharges in the hippocampus one can observe a clear depression of all EEG rhythms (Fig. 2B). In the presence of the EEG shifts mentioned above, conditioned signals cease to activate feeding behavior. Lack of activation of food-motor behavior in response to electrical stimulation of the hippocampus, as well as the lack of efficiency of conditioned signals, in the sense of triggering the relevant behavior, should indicate that at the same time there does not occur triggering of the relevant motivational processes. This fact also questions the possibility of localization in this structure of the memory trace by activation of which it is possible to reproduce the significance of a conditioned signal.
A comparison of the dynamics of elaboration of instrumental alimentary reflexes with sound discrimination has shown that in the first three learning sessions the experimental group animals lag behind the controls by the percentage of correct responses (Fig. 5), although both groups reach 100% discrimination of conditioned signals only at the fourth learning session. This indicates that preliminary postconvulsive depression of the hippocampal theta rhythm has a stronger effect on the elaboration of generalized instrumental alimentary reflexes than on the subsequent elaboration of discrimination of conditioned sounds. This conclusion follows from the supposition that in the case of two choices the level of 50% correct responses shows that sound stimuli acquire a signal meaning, so the choice of feeders is quite random, whereas the further increase of correct responses must reflect discrimination of conditioned signals.
There was no difference in the rate of extinction of conditioned reflexes either; in both cases during the first three days one could observe an acute daily extinction with the restoration of conditioned reflexes on the following day, while after a four-day work chronic extinction is reached.
Influence of functional elimination of the hippocampus on learning and memory
1. Influence of functional elimination of the hippocampus after daily sessions on the elaboration of instrumental alimentary reflexes.
One of the methods of functional elimination of the cortical structures is the induction of epileptiform discharges (Kesner and Doty 1968; McDonough and Kesner 1971; Vardaris and Schwartz 1971; Shinkman and Kaufman 1972). This method was employed by us to study the influence of functional elimination of the hippocampus on learning and memory. Experiments were carried out in four groups of animals. In the first group were animals in which the influence of post-training depression of the hippocampal theta rhythm on learning and memory was studied. Functional elimination of the hippocampus was effected by means of periodic induction of local epileptiform discharges. As this procedure lasted 2 hours (during this time epileptiform discharges were induced at 2-3 min intervals) after daily sessions of learning, it may be considered that in this case we had rather a long functional elimination of the hippocampus, embracing most of the initial part of the memory trace consolidation phase. It might be assumed that if the hippocampus is indeed a privileged structure in the organization of memory traces during learning, then its functional elimination would be particularly effective in the first hours following a series of pairings of conditioned signals with food reinforcement. However, the effect proved to be insignificant. Though, as shown above, the functional elimination of the hippocampus by the induction of repeated epileptiform discharges within two hours after each learning session retarded the elaboration of instrumental alimentary reflexes to two feeders with sound discrimination, such an effect is more pronounced in the first learning sessions, i.e. during the initial stages of elaboration of instrumental alimentary reflexes (Fig. 6). But in this situation further differentiation of conditioned signals is performed almost normally.
2. Influence of short-lasting functional elimination of the hippocampus following each combination of conditioned signals with food reinforcement on the elaboration of instrumental alimentary reflexes.
In this series the experiments were performed on three groups of animals. In the first group the hippocampal epileptiform discharges were evoked in a 1 min, in the second group in 25 sec and in the third immediately after each pairing of conditioned signals with food reinforcement. Analysis was made of the data obtained only in those cats in which the local epileptiform discharges were obtained in the hippocampus but they did not spread considerably to the other brain structures Irrespective of any of the above intervals between the pairing of a conditioned stimulus with unconditioned and the functional elimination of the hippocampus by epileptiform discharges no delay occurred in the elaboration of instrumental alimentary reflexes (Fig. 7). On the contrary, in the experimental animals, as compared with the controls, a noticeable facilitation of elaboration of conditioned reflexes was observable. Thus, for example, if in the control animals 100% discrimination of conditioned sounds is achieved on the fourth day, then in the animals with the hippocampal epileptiform discharges induced within a minute of the pairing of conditioned and unconditioned signals it is achieved on the third day of experimentation (Fig. 7A). The same was noted in the cases when the epileptiform discharges in the hippocampus were induced with 25 sec delay and immediately after the pairing of signals (Fig. 8).
Hence, it may be concluded that the functional elimination of the hippocampus by induction of epileptiform discharges even after each pairing of a conditioned signal with an unconditioned one does not lead to the delay of elaboration of instrumental alimentary reflexes to two feeders. A facilitatory effect of hippocampal epileptiform discharges is in need of additional analysis (see debates around the report of prof. T. Oniani, which was conducted in 1983, at the 8th Symposium known as "Gagra Talks").
3. Influence of functional elimination of the hippocampus on extinction of conditioned alimentary reflexes.
In this series of experiments functional elimination of the hippocampus by induction of local epileptiform discharges, in one group of animals, was effected for 2 hours following a daily session on extinction. The data are described above in the section on the influence of post-training depression of the hippocampal theta rhythm on extinction. As has been stated, such a procedure does not delay the process of extinction of instrumental reflexes with sound discrimination.
In another group of animals local epileptiform discharges in the hippocampus were induced after every run to the feeder in response to this or that conditioned signal without food reinforcement. Naturally, extinction in this case, too, was started after a stable elaboration of instrumental alimentary reflexes to two feeders. In some cats epileptiform discharges in the hippocampus were induced after 1 min and in others, immediately after each run to the feeder. Acute as well as chronic extinction in both groups occurred more rapidly than in controls (Fig. 9). Thus, functional elimination of the hippocampus by induction of epileptiform discharges causes facilitation of an extinction process rather than its retardation.
4. Influence of functional elimination of the hippocampus on conditioned delayed reactions.
For this purpose after 100% discrimination of conditioned sounds to two feeders delayed reactions were measured in the animals. Maximum delay time varied from cat to cat within 1-10 min. Experiments with the study of the influence of functional elimination of the hippocampus were carried out on cats in which the maximum of delayed reactions was not less than 5 min. In different series of experiments the hippocampal epileptiform discharges were induced l0, 5, 2 sec and immediately after the cessation of a directing conditioned stimulus. Hippocampal epileptiform discharges induced 10 sec after the cessation of a directing conditioned signal were found to result in no disturbances of delayed reactions (Fig. 10A), whereas those induced at 5 (Fig. 10B) or 2 sec (Fig. 10C) interval bring about disturbances in the correct choice of feeders not only during maximal but also during minimal delays.
Effect of abolishment of the hippocampal theta rhythm by PSD on the elaboration of instrumental alimentary reflexes
Abolishment of hippocampal theta rhythm by recurrent induction of epileptiform discharges and study of the effect of this procedure on learning may lead to controversy. As a matter of fact, hippocampal epileptiform discharges produced by its direct electrical stimulation, resulting in the suppression of hippocampal theta rhythm, may affect the integrative activity of the brain in some other ways too. It has been earlier demonstrated that during the development even of local epileptiform discharges induced by hippocampal electrical stimulation there occurs non-specific activation of the brain, leading to desynchronization of neocortical electrical activity (Oniani and Nachkebia A. 1982). It is quite possible that such non-specific activation of the brain may positively influence the process of learning (see Hebb 1949), masking thereby the negative effect of the hippocampal theta rhythm deficit. The interpretation of the data obtained during epileptiform discharge induced suppression of hippocampal theta rhythm may entail difficulties because epileptiform discharges may result not only in the suppression of theta rhythm but also in the functional elimination of the hippocampus, which is an important link of the limbic system. And the disturbance of the integrity of the limbic circuit may affect the learning process. This may account for the initial impairment of the elaboration of instrumental alimentary reflexes taking place both during preliminary postconvulsive depression of hippocampal theta rhythm and its depression in the intersession periods.
Proceeding from these considerations, in order to study the significance of hippocampal theta rhythm in the organization of learning and memory it is advisable to find more appropriate procedures which would not entail such difficulties. It is of course understandable that the hippocampal theta rhythm has to be abolished precisely in the phase of memory trace consolidation, as this rhythm has been reported to perform its function most optimally during the transition of short-term or recent memory into long-term, i. e. in the phase of consolidation.
During the elaboration of instrumental alimentary reflexes in many-day learning sessions of memory trace consolidation the phases in which the acquired information become stable and short-term memory is converted into long-term coincide with the intersession periods. The optimal period of the consolidation phase must last several hours after each session. By that time the cats - already satiated with the reinforcing portions of meat - are calm and develop a full sleep-wakefulness cycle. In this cycle the dynamics of hippocampal electrical activity undergoes regular changes. Against the background of motor activity (walking) which is rare in satiated cats in a usual experimental chamber, a moderate hippocampal theta rhythm may appear in parallel to desynchronization in neocortical electrical activity (Fig. 11A). In the phase of wakefulness the cats usually either sit or lie with their heads up, the regular hippocampal theta rhythm being absent. Slow-wave sleep is characterized by the occurrence of high-amplitude, slow potentials (in the range of delta and subdelta rhythms) in the hippocampus (Fig. 11B). Naturally, in this case slow waves appear in the neocortex. In PS during desynchronization of the electrical activity in the neocortex and rapid eye movements there develops a progressive hippocampal theta rhythm (Fig. 11C).
From the above figure it is obvious that during the learning intersession periods, i.e. in the phase of consolidation of an acquired information, the hippocampal theta rhythm, reflecting the intensive functioning of the septo-hippocampal system, mainly develops in PS. If PSD is effected by nonemotional awakenings of the animal, the hippocampal theta rhythm is disturbed and does not recover during the waking episodes (Fig. 12.1, 12.2). Hippocampal theta rhythm is lacking during those more or less long waking episodes which can arise during prolonged PSD in learning intersession periods. PSD is therefore a good technique for studying the effect of abolishment of hippocampal theta rhythm on memory trace consolidation.
Experiments on the effect of abolishment of hippocampal theta rhythm by PSD in the learning intersession periods were carried out on cats. Such cats were selected which had a well-pronounced diurnal sleep-wakefulness cycle with a considerable amount of PS (13-15% of the total time). The learning sessions on elaboration of instrumental alimentary reflexes to two feeders lasted from 10 a.m. to 12 a.m. After the learning session the cats were kept in the experimental chamber, where they soon went to sleep. In the first series of experiments-only 6 hr PSD was performed after the learning session (from 12 a.m. to 6 p.m.). Then the cats were taken to the vivarium, where they could take a whole course of cycle till the next session at 10 a.m. of the following day. In the second series of experiments the PSD was done by awakenings throughout the whole intersession time.
Neither 6 hr nor 24 hr PSD, resulting in the abolishment of the hippocampal theta rhythm characteristic for PS, have proved to have a noticeable effect on the learning process. Instrumental alimentary reflexes to two feeders based on sound discrimination were equally well elaborated both in the experimental and control groups (Fig. 13).
Arguments against the above conclusion on the non-participation of the hippocampal theta rhythm development in memory trace consolidation can be found in the observation that when inducing epileptiform discharges and administering PSD following learning sessions this rhythm is present and performs its function in the course of learning sessions. These arguments are demolished however by the fact that against the background of preliminary postconvulsive depression of the hippocampal theta rhythm only slight retardation of instrumental alimentary reflex elaboration is observed, but that is in the initial stages of learning. Yet, in order to verify this assumption it is advisable to carry out experiments on septal animals and under conditions of permanent elimination of the hippocampal theta rhythm.
Influence of septal lesions on general behavior, the elaboration and extinction of instrumental conditioned alimentary reflexes and on delayed reactions
1. Influence of septal lesion on motivational-emotional reactions and the sleep-wakefulness cycle.
As is known, for the hippocampal theta rhythm to be abolished it is sufficient to damage the medial nucleus of the septum where the pacemaker of this electrical activity is localized (Petsche and Stumpf 1960; Petsche et al. 1962; Vinogradova and Braznik 1978). However, because of the small size of the medial nucleus electrocoagulation frequently spreads to the adjacent regions of the septum as well. On the basis of the character of the effects obtained by septal lesions and their comparison with the data of morphological control of the lesion extent, the experimental animals were subdivided into three groups. The first group consisted of animals in which a more or less pure lesion was placed in the medial nucleus. The second group consisted of animals in which in addition to the medial septum the lateral nucleus was also partially disturbed. Cats with massive lesion of the whole septum fell into the third group.
In all experimental cats belonging to the above three groups the electrical activity of the hippocampus was altered equally, as evidenced by the fact that the hippocampal theta rhythm was entirely abolished during the performance of motivational-emotional reactions .against the background of wakefulness as well as during the development of PS (Fig. 14.1, 14.2). Behavioral reactions and the structure of the sleep-wakefulness cycle altered in different ways. Food motivation, expressed in hyperphagia, was particularly enhanced in those animals in which the entire septum was lesioned. Sometimes the amount of food intake increased 2-3 times. The so-called "septal syndrome" described in rats (Brady and Nauta 1955; Brady 1958; King 1958; Brown and Remley 1971), i.e. rise of sensitivity to stimuli to which the animal had earlier reacted slightly or paid no attention, post-operation inability to stay in a certain situation during the same period of time as before the surgery, development of perseverative movements, etc., developed in cats only in cases where the lateral nucleus was lesioned in addition to the medial septum. Depending on the extent of lesion, the structure of the sleep-wakefulness cycle altered in different ways. The structure of the sleep-wakefulness cycle and the percentage of different phases changed considerably only in those cats that suffered extensive destruction of the septum, as evidenced by an increase of the amount of wakefulness and decrease of PS. Destruction of only the medial nucleus resulted in no noticeable changes.
2. Influence of septal lesion on previously elaborated instrumental alimentary reflexes.
In this series of experiments study was made of the influence of septal lesion on previously elaborated instrumental conditioned alimentary reflexes. Under these experimental conditions the control animals without septal lesion developed a pronounced hippocampal theta rhythm in response to a conditioned signal and during approach to the feeders. It might be assumed that this theta rhythm is in some way causally associated with the retrieval of conditioned reflexes, i.e. readout of memory traces. However, in cats with lesions only in the medial nucleus of the septum, in which the hippocampal theta rhythm disappeared altogether, the previously elaborated conditioned reflexes were not found to be altered, i. e. 100% discrimination of conditioned sounds was maintained. This indicates that development of the hippocampal theta rhythm is not necessary for the retrieval of conditioned alimentary reflexes.
A different picture was observed if, in addition to the medial nucleus of the septum, the lateral one was also lesioned. In these cats, in parallel to the development of the septal syndrome, the elaborated conditioned reflexes were disturbed and we had to reelaborate them, this procedure requires about the same number of pairings as their elaboration for the first time in intact animals (Fig. 15). Thus, comparison of the data obtained in the above two groups of animals enabled us to conclude that it is not the abolishment of the hippocampal theta rhythm that leads to the disturbance of memory traces, but a deficit of the function of the lateral nucleus of the septum or elimination of the influences that the hippocampus may exert via the septum on other brain structures.
3. Influence of septal lesion on the elaboration of instrumental alimentary reflexes.
In a subsequent series of experiments study was made of the influence of septal lesion on the elaboration of conditioned alimentary reflexes to two feeders. The effect in this case, too, depended on the extent of the lesion. In cats with restricted lesion of the medial nucleus, in which the hippocampal theta rhythm had been abolished, conditioned alimentary reflexes were as readily elaborated as in controls (Fig. 16). If the lesion involved the lateral nucleus as well, then elaboration was considerably delayed (Fig. 17). This fact indicates that the change of the learning process following septal lesions is not due to the abolition of the hippocampal theta rhythm but to a deficit in the function of the lateral nucleus of the septum or to the elimination of the influences that the hippocampus exert via septum upon other brain structures.
4. Influence of septal lesion on conditioned delayed reactions.
Measurement of delayed reactions was begun against the background of 100% discrimination of conditioned stimuli to two feeders. After this one or another conditioned signal was given for 10 sec and the animal was released from the starting section only some time after the cessation of conditioned stimuli. In this way the maximum delay time was determined, after which the cats could still approach the signaled feeder correctly and errors did not exceed 5-l0%: after the maximum duration of delayed reactions was established, a lesion was placed in the septum and, after some days, the duration of conditioned delayed reactions was again checked. The cats with septal lesions proved incapable of performing conditioned delayed reactions. It should be also emphasized that the delayed reactions were equally disturbed both in cats with a restricted lesion of the medial nucleus of the septum and in those with a lesion involving the lateral nucleus. Prior to the lesion these cats accomplished almost 100% discrimination of conditioned sounds even at 5 min delays. Following lesion of the medial septum in the same cats at 5 min delays a complete disturbance of the discrimination of conditioned signals is observable. Cats with septal lesions also leave the starting section, but their reactions to conditioned signals are no more than 50% correct (Fig. 18), which may be considered as an absolute error in the case of two choices. Their choice of feeders is random even when they are released from the starting section 2-5 sec after the cessation of conditioned signals. This indicates that even a restricted lesion of the medial nucleus of the septum renders the animals incapable of performing conditioned delayed reactions in general, while discrimination of conditioned signals during the action of conditioned signals is well preserved.
As indicated above (Fig. l5) in the cases when the lateral nucleus of the septum is also damaged we have to re-elaborate sound discrimination, but restoration of conditioned delayed reactions does not occur.
Discussion
1. Influence of postconvulsive depression of the hippocampal theta rhythm on learning and memory
At present the fact is beyond doubt that the development of the hippocampal theta rhythm is an electrographic manifestation of the high functioning of the septo-hippocampal system (Petsche and Stumpf 1960; Petsche et al. 1962). On the other hand, the statement is very common according to which the dynamics of the hippocampal theta rhythm reflects different steps of memory organization (Grastyan et al. 1959; Adey 1961, 1962, 1966; Morrel 1961; Elazar and Adey 1967; Pickenhaim and Klingberg 1967). Proceeding from this, abolishment of the hippocampal theta rhythm through a variety of influences disturbing the normal functioning of the septo-hippocampal system is expected to have a negative effect on learning and memory. There are indications in the literature (Hostetter 1968; Barcik 1970; McGaugh 1973) that the amnesic action of electroconvulsive shock results from depression of the hippocampal theta rhythm. This statement is difficult to prove since an electroconvulsive shock may cause depressive changes in other brain structures, as well as depressing the hippocampal theta rhythm, and this may also influence the organization of memory. It would be more convincing if one could study the effect of the depression of the hippocampal theta rhythm on learning and memory without disturbing the functional state of other brain structures.
On the basis of the foregoing it may be concluded that both preliminary depression of the hippocampal theta rhythm and its depression during learning intersession periods retard but slightly (in initial stages) the elaboration of instrumental alimentary reflexes. But it turned out that the retardation is not due to the absence of the hippocampal theta rhythm in these experiments. This follows from the fact that elimination of hippocampal theta rhythm development during the learning intersession periods by way of PSD non-emotional awakening of the animal does not retard the elaboration of conditioned reflexes in the initial stages either. This finding indicates that development of the hippocampal theta rhythm per se is not of specific significance in memory trace consolidation and that the negative effect of postconvulsive theta rhythm depression must be due to the functional state changes either in the hippocampus itself or in the mesodiencephalic structures.
2. Influence of septal lesions on learning and memory.
To permanently abolish the hippocampal theta rhythm the best way is to lesion the medial nucleus of septum where its pacemaker mechanism is localized (Petsche and Stumpf 1960; Petsche et al. 1962). However, in this case, evaluation of the significance of the hippocampal theta rhythm will be hindered by the fact that the septum - as an important structure of the limbic system - may influence the course of events in other ways too. It is well known that lesion of septum leads to conspicuous changes in the motivational-emotional state of animals. Thus, for example, following septal lesion in rats and mice there develops rage and other signs of hyperemotionality which has been termed the "septal syndrome" (Brady and Nauta 1955; Brady 1958; King 1958; Brown and Remley 1971). Development of hyperemotionality following septal lesion has been described in cats too (Moore 1964). It has not been observed in hamsters (Sedetz et al. 1970), and monkeys (Buddington et al. 1967; Butters and Rosvold 1968). With regard to the relation between the hippocampal theta rhythm and learning and memory it is of particular interest that a septal syndrome does not develop in the case of a restricted lesion of the medial septum (Clody and Cariton 1969). On the contrary, in this case, one can observe an attenuation of the emotionality of the animal. In the opinion of Isaacson (1976) in the septum there is a topographic localization of function with regard to the development of the septal syndrome.
In our experiments with cats the septal syndrome was obtained only if the lesion involved the lateral septum as well. A local lesion restricted to the medial septum caused the abolishment of the hippocampal theta rhythm without causing the septal syndrome. In this respect our data confirm those of Clody and Cariton (1969) obtained in rats. Apart from other components of the septal syndrome during extensive septal lesion in our experiments the development of hyperphagia, i. e. increase of food motivation, was observed. This should necessarily be taken into account when studying the influence of septal lesion on the elaboration and extinction of instrumental alimentary reflexes.
In our experiments a restricted lesion in the medial septum, leading to the disappearance of the hippocampal theta rhythm, without a parallel development of the septal syndrome, had no effect either on the elaboration of conditioned instrumental alimentary reflexes to two feeders or on the extinction of these reflexes. Only when the lateral septum was also damaged and, in addition to the disappearance of the hippocampal theta rhythm the "septal syndrome" also developed, there was an effect on learning which consisted in difficulties with the extinction of instrumental alimentary reflexes. Difficulties with the extinction of conditioned alimentary reflexes following septal lesions have been reported by other authors too (Shwartzbaum et al. 1964; Pubols 1966; Butters and Rosvold 1968). However, acceleration of extinction of a conditioned avoidance reaction has been also noted following septal lesion (Butters and Rosvold 1968). The dependence of the effect of septal lesion on the type of conditioned reflex and on the experimental situation clearly indicates that in the present case we are not dealing with changes of the memory mechanism underlying learning, but with the development of processes that hinder the optimal operation of these mechanisms. In our experiments such processes might be the development of hyperphagia and hyperactivity.
The septal lesion may exert the most significant influence on the conditioned delayed reactions which are completely abolished in the lesioned animals. Moreover, disturbance in the delayed reactions is observed both in cats with destruction of both the medial and lateral parts of the septum and in cats with lesions only in the medial septum. This fact may be interpreted in the light of the hypothesis (Konorski 1967; Oniani 1979) according to which delayed conditioned reactions are based on short-term memory in "pure form" (nonconsolidated form). In this case they may depend upon a long-term elaborated mechanism of the conditioned reflex and, therefore, there is no need for transfer to long-term memory. It has been assumed that the neurophysiological basis of short-term memory in "pure form" is the reverberation of excitation in the functionally organized nervous circuits and subsequent posttetanic potentiation of excitation conductance in them (Konorski 1967; Oniani 1979). The limbic system seems to play an important role in the organization of these nervous circuits and, therefore, a lesion that interrupts the integrity of this circuit (in our case lesion of the septum which holds a strategic position in the limbic system) leads to a disturbance of conditioned delayed reactions.
From the foregoing it follows that development of synchronization of the hippocampal electrical activity in the range of theta rhythm is not indispensable to learning and fixation of memory traces.
3. Influence of functional elimination of the hippocampus on learning and memory.
The view is prevalent in the neurophysiological literature that the hippocamapal theta rhythm is relevant to the organization of learning and memory, which is a logical extension of the statement on the significance of the hippocampus itself in these processes. This statement was formulated solely on the basis of clinical observations (Penfield and Milner 1958; Milner 1968) according to which patients with a lesioned hippocampus fail to remember recent events, i.e. recent memory does not convert into long-term one. Experimental studies carried out on animals with a lesioned hippocampus have accumulated a number of contradictory facts, but the investigators engaged in the search of electrophysiological correlates of learning and memory have apparently been more impressed by the clinical observations.
In order to discuss the question of the significance of the hippocampus itself in the organization of learning and memory the facts obtained by studying the influence of functional elimination of the hippocampus are no doubt important. It is known that functional elimination of cortical structures can be achieved by inducing convulsive electrical activity in them (Kesner and Doty 1968; McDonough and Kesner 1971; Vardaris and Schwartz 1971; Shinkman and Kaufman 1972). This method can be most successfully applied to the hippocampus, since by dosed electrostimulation one can induce in it local epileptiform discharges which do not spread considerably to adjacent brain structures. It is well known that the hippocampus, having a well organized internal structure, is divided into areas and segments between which in normal conditions there is a coordinated interaction (Andersen et al. 1971; 1973). During the development of epileptiform activity this interaction is disturbed, and as a result the hippocampus becomes incapable of receiving and processing input information. This equals to a functional elimination of the hippocampus.
In estimating this method of functional elimination of the hippocampus it can be assumed that repeated electrical stimulation could result in the development of the kindling phenomenon (Goddard et al. 1969), which in turn would affect learning and memory. However, such an assumption seems untenable for at least three reasons. First, the parameters of electrical stimulation in our experiments were threshold for the epileptiform discharges to be induced and not subthreshold as is needed for kindling. Second, the intervals between repeated stimulations were much shorter (2-3 min), compared with those required for the development of the kindling process, and third, the fact is important that reduction of the hippocampal stimulation threshold for the epileptiform discharges to be induced in it was not observed in our experiments either in the first session, or in the subsequent ones. Reduction of the stimulation threshold is known to be one of the features of the development of the kindling process (Goddard et al. 1969). We have used this method of functional elimination of the hippocampus in two series of experiments. In the first series during the elaboration of instrumental alimentary reflexes to two feeders after each daily session epileptiform discharges were periodically induced in the hippocampus for two hours at 2-3 min intervals. In the other series of experiments functional elimination of the hippocampus by means of inducing in it epileptiform discharges was effected after each combination of a conditioned signal with food reinforcement. No difficulty with the elaboration of instrumental alimentary reflexes was observed in the first and second cases. On the contrary, there was a marked facilitation of discrimination of conditioned signals. All this makes us think that the hippocampus is hardly a unique structure playing a decisive role in the consolidation of memory traces. On the other hand, the hippocampus, as one of the most important structures of the limbic circuit, must be of great significance in the organization of such a form of learning as extinction of an instrumental alimentary reflex, as also in the organization of short-term memory in "pure form". The effect of a lateral septal lesion, which hinders the extinction of an instrumental alimentary reflex, may be considered a deficit of the descending influence of the hippocampus on mesodiencephalic structures, since efferent pathways of the hippocampus are disrupted. The participation of the hippocampus in the organization of short-term memory in "pure form" is indicated by the disturbance of conditioned delayed reaction when it is functionally eliminated by epileptiform discharges after the delivery of directing conditioned signals.
On the other hand, special consideration should be given to the facilitatory influence of hippocampal epileptiform discharges when they were induced after each pairing of a conditioned signal with food reinforcement.
Parallel studies of the dynamics of neocortical electrical activity yield useful information on the reasons for this phenomenon. It has been demonstrated (Oniani 1980) that, against the background of hippocampal epileptiform discharges, the electrical activity in the neocortex undergoes a diffuse desynchronization, as it does during stimulation of the activating nonspecific structures of the mesodiencephalon. This phenomenon manifests itself well in intact animals, but is partially maintained even after section of the brain stem at the precollicular level. This fact indicates that during the development of epileptiform discharges in the hippocampus there occurs excitation of nonspecific activating structures of the mesodiencephalon which, in turn, exert an activating influence on the neocortex. The excitatory influence of the hippocampus on nonspecific activating structures of the brain stem is displayed also during electrical stimulation at levels below those causing convulsive activity. It has been demonstrated that electrical simulation of the hippocampus produces excitatory post-synaptic potentials (Grastyan et al. 1959) in the neurons of the mesencephalic reticular formation, and that a high-frequency electrical stimulation of the hippocampus even without the development of convulsive activity leads to desynchronization of electrical activity in the neocortex (Oniani 1980).
In the light of this evidence the data reported on the facilitation of learning by electrical (Chow 1961; Stein and Chorover 1968; Destrade and Cardo 1974; Soumireu-Mourat et al. 1975; Gralewicz 1976; Wetzel et al. 1977) and chemical (Ott 1979) stimulation of the hippocampus and septum have been given a satisfactory interpretation. In the above cases diffuse activating structures of the mesodiencephalon were probably involved in activity, and this led to the facilitation of learning. It has been shown that an increased level of activity of mesodiencephalic activating structures via electrical stimulation no doubt has an important effect on the facilitation of learning (Bloch et al. 1970; Kedia 1970; Iljuchenok 1972; Belenkov 1977). This is indicated also by the activating effect of such pharmacological agents as strychnine, amphetamine, etc., on learning.
In this aspect one can consider also the data reported by McGaugh (McGaugh 1966; Landfield et al. 1972) and others showing a correlation between the prominence of the hippocampal theta rhythm and the rate of learning.
The prominence of the hippocampal theta rhythm reflects the level of activation of mesodiencephalic structures. It increases during a nonspecific activation of the brain, as well as in response to an increased activity of specific motivating structures (Oniani 1980). From this it follows that the animals with a pronounced hippocampal theta rhythm, i.e. with a high level of activity of mesodiencephalic structures would learn more easily than those with an ill-pronounced theta rhythm, which is indicative of a low level of brain activity. Such an interpretation of the facts is in agreement with Hebb's theory (Hebb 1949) on the significance of the level of reticular activation for an optimal dynamics of informational processes in the brain.
Delayed conditioned reactions proved most sensitive to the disturbance of the integrity of the septo-hippocampal system. They are completely disturbed both after medial septum lesion, resulting in the abolishment of the theta rhythm, and during functional elimination of the hippocampus at short intervals after the cessation of directed conditioned signals. This fact attests to the significance of the hippocampus and intimately related structures in the regulation of short-term operative memory (Olton 1978; Olton et al. 1982), underlying the regulation of delayed conditioned reactions (Konorski 1967; Oniani 1979).
In conclusion an analysis of the above facts does not confirm the hypothesis that the hippocampus and its theta rhythm play an exclusive role in the organization of learning and memory. In all probability, the hippocampus is involved in the regulation of these processes in an intimate interrelation with other structures of the limbic system, and especially with the activating and motivating structures of the mesodiencephalon.
Work was reported in 1983 at the 8th Symposium known as "Gagra Talks" (Gagra, Georgia). Unfortunately, the collection of papers of the Symposium has not been published. The questions considered in the presentation and its discussion are still of topical importance and mainly deal with the elucidation of the role of septo-hippocampal system and various phases of the sleep-wakefulness cycle in the organization of learning and memory. We think it reasonable to publish them unrevised in this issue. Considered in the report and its discussion is a complex of problems on the neurobiology of learning and memory. These questions which have subsequently been subjected to additional experimental analysis and interpretation, taking into account the recent data reported in special literature, will be dealt with in other publications.
References
Adey, W.R. Studies of hippocampal electrical activity during approach learning In: I.F Delafresnaye (Ed.), Brain Mechanisms and Learning. Springfield, Thomas, 1961, III: 577-588.
Adey, W.R. EEG studies of hippocampal system in the learning processes. In: Physiologie de l'hippocampe. Paris, Centre national de Recherche Scientifique, 1962: 203-222.
Adey, W.R. Neurophysiological correlates of information and storage in brain tissue. In: E. Stellar, J.M. Sparaque, (Eds), Progress in Physiological Psychology. N.Y Acad. Press, 1966, I, I.
Andersen, P., Bliss, T.V.P., Strede, K.K. Lamellar organization of hippocampal excitatory pathways. Exptl. Brain Res., 1971, 13, 2: 222-238.
Andersen, P., Bland, B.I., Dudar, J.D. Organization of the hippocampus output. Exptl. Brain Res., 1973, 17, 2: 152-168.
Andersen, P., Wyrwika, W. The elicitation of a drinking motor conditioned reaction by electrical stimulation of the hypothalamic "drinking area" in the goat. Acta physiol. Scand., 1957, 41: 194-198.
Anschel, C., Anschel, S. The effects of hippocampal seizure on self-stimulation in squirrel monkeys. Electroenceph. Clin. Neurophysiol., 1969, 26: 436-443.
Barcik, I.D. Hippocampal after-discharges and conditioned emotional response. Psychol. Sci., 1970, 20: 297-299.
Bekhterev, V. Demonstration eines Gehirns mit Zerstorung der vorderer und inneren Teile der Hirnrinde beider Schlafenlappen. Neurol. Zentralblatt., 1900, 19: 990-991.
Belenkov, N.I. The role of subcortical formations in conditioned reflex activity. In: Handbook of Physiology of Higher Nervous Activity. Nauka. Moscow, 1977: 232-239 (in Russian).
Bennelt, T.L. Bioelectrical activity of the hippocampus. In: Functional Significance of Electrical Processes of the Brain. Nauka, Moscow, 1977: 232-239 (in Russian).
Bloch, V. Facts and hypotheses concerning memory consolidation processes. Brain Res., 1970, 24: 561-575.
Bloch, V., Deweer, B., Hennevin, E. Suppression de l'amnesie retrograde et consolidation dum apprentiscageo' essai unique par stimulation reticulaire. Physiol. Behav., 1970, 5: 1235-1241.
Brady, J. L. The paleocortex and behavioral motivation. In: Harlaw and Woolsey (Eds.), Biological and Biochemical Bases of Behavior. Univ. of Wisconsin Press. Madison, 1958: 193-235.
Brady, J.L., Nauta, W.I. Subcortical mechanisms in control of behavior. J.Comp. Physiol. Psychol., 1955, 48: 412-420.
Brown, G.E., Remley, N.R. The effects of septal and olfactory bulb lesion in stimulus reactivity. Physiol. Behav., 1971, 6: 479-501.
Buddington, R.W., King, F.A., Roberts, L. Emotionality and conditioned avoidance responding in the squirrel monkey following septal injury. Psych. Science, 1967, 8: 195-196.
Butters, N., Rosvold, H.E. Effects of septal lesions on resistance to extinction of a delayed alternation in monkeys. J. Comp. Physiol. Psychol., 1968, 66: 389-395.
Carl, P.J. Effects of hippocampectomy in a one-trial electroconvulsive shock paradigm. Physiol. Psychol., 1979, 7: 53-58.
Chow, K. L. Effect of local electrographic afterdischarges on visual learning and retention in monkey. J. Neurophysiol., 1961, 25: 391-399.
Clody, D.E., Cariton, P.L. Behavioral aspects of lesions of the medial septum of rats. J.Comp. Physiol. Psychol., 1969, 67: 344-351.
Delgado, S.M.R., Roberts, W.W., Miller, N.E. Learning motivated by electrical stimulation of the brain. Amer. J. Physiol., 1954, 179: 587-593.
Destrade, C., Cardo, B. Effects of post-trial hippocampal stimulation on time-dependent improvement of performance in mice. Brain Res., 1974, 78: 447-454.
Douglas, R.J. The hippocampus and behavior. Psychol. Bull., 1967, 67: 416-442.
Elazar, Z., Adey, W.R. Electroencephalographic correlates of learning in subcortical structures. Electraenceph. Clin. Neurophysiol., 1967, 23: 306-319.
Fishbein, W. Interference with conversion of memory from short-term to long-term storage by partial sleep deprivation. Commun. Behav. Biol., 1970, 5: 171-175.
Fishbein, W. Disruptive effects of rapid eye movement sleep deprivation on long-term memory. Physiol. Behav., 1971, 6: 279-282.
Fishbein, W., McGaugh, I.L., Swarz, I.R. Retrograde amnesia: Electroconvulsive shock effects after termination of rapid eye movement sleep deprivation. Science, 1971, 172: 80-82.
Goddard, C.V., McIntyre, D.C., Leech, C.K. A permanent change in brain function resulting from daily electrical stimulation. Exp. Neurol., 1969, 25: 295-330.
Gralewicz, S. Electrical stimulation of the hippocampus and acquisition of the conditioned avoidance response in shuttle-box in cats. Acta Neurobiol. Exp., 1976, 36: 640-654.
Grastyan, E., Lissak, K., Madarasz, I., Donhoffer, M. Hippocampal electrical activity during the development of conditioned reflex. Electroenceph. Clin. Neurophysiol., 1959, II, 3: 409-430.
Green, I.G., Arduini, A.A. Hippocampal electrical activity in arousal. J. Neuraphysiol., 1954, 17: 533-557.
Green, I.D., Maxwell, D.S., Schindler, W.S., Stumph, C. Rabbit EEG "theta rhythm": its anatomical source and relation to activity in single neurons. J. Neurophysiol., 1960, 23: 403-420.
Green, I.D., Petsche, H. Hippocampal electrical activity. II. Virtual generators. Electroenceph. Clin. Neurophysiol., 196l, l3: 847-853.
Grossman, S.P. Direct adrenergic and cholinergic stimulation of hypothalamic mechanisms. Amer. J. Physiol., 1962, 202: 872-882.
Hebb, D.O. The Organization of Behaviar. A Neurophysiological Theory. New York, Willey, 1949.
Hirano, T. Effect of functional disturbances of the limbic system on the memory consolidation. Jap. Psychol. Res., 1965, 7: 171-182.
Hostetter, G. Hippocampal lesions in rats weaken the retrograde amnesic effect of ECS. J. Comp. Physiol. Psychol., 1968, 66: 349-353.
Iljuchenok, P.Y. Pharmacology of Behavior and Memory. Nauka, Novosibirsk, 1972 (in Russian).
Isaacson, R.L. The Limbic System. Plenum Press, New York-London, 1976.
Jasper, H.H., Ajmone-Marsan, C. A Stereotaxic Atlas of the Diencephalon of the Cat. Ottawa Nat. Res. Council, Canada, I954.
Jung, R., Kornmuller, A. Eine methodik der ableitung localisierten potentialshwenkkugen aus subcorticalen Hirngebieten. Arch. Psychiat. Nervenkr., 1938, 109: 1-30.
Kedia, I. A. The influence of partial bilateral lesion of the mesencephalic reticular formation on the conditioned reflex activity. Bull. Georg. Acad. Sci., 1970, 57, 1: 189-192 (in Russian).
Kesner, R.P., Doty, R.W. Amnesia produced in cats by local seizure activity initiated from the amygdala. Exptl. Neurol., 1968, 21: 58-68.
King, E.A. Effects of septal and amygdala lesions on emotional behavior and conditioned avoidance responses in the rat. Science, 1958, 128: 655-656.
Konorski, M.D. Integrative Activity of the Brain. The University of Chicago Press, 1967.
Landfield, P.W., McGaugh, J.L., Tusa, R.J. Theta rhythm: A temporal correlate of memory storage processes in the rat. Science, 1972, 175: 87-89.
McDonough, I.H., Kesner, R.P. Amnesia produced by brief electrical stimulation of amygdala or dorsal hippocampus in cats. J.Comp. Physiol. Psychol., 1971, 77: 171-178.
McGaugh, J.L. Time dependent processes in memory storage. Science, 1966, 153: 1351-1358.
McGaugh, J.L. Impairment and facilitation of memory consolidation. Activ. Nerv. Super., 1972, 14: 64-74.
McGaugh, J.L. The search for the memory trace. Ann. N.Y. Acad. Science, 1973, 193: 112-123.
Mering, T.A., Mukhin, E.I., Pigarjova, M.L. The hippocampus and inhibitory processes. Zhurn. Visshei Nervn. Dejat., 1972, 22: 917-923 (in Russian).
Milner, B. Visual recognition and recall after right temporal lobe excision in man. Neuropsychologia, 1968, 6: 19l-202.
Morrel, F. Effect of anodal polarization on the firing pattern of single cortical cells. Annals of N.Y. Acad. Science, 1961, 92: 860-876.
Moore, R.Y. Effects of some rhinencephalic lesions on retention of conditioned avoidance behavior in cats. J. Comp. Physiol. Psychol., 1964, 53: 540-548.
Olton, D.S. The function of septo-hippocampal connections in spatially organized behavior. In: Functions of Septo-Hippocampal System. Ciba Foundation Symposium. Elsevier. Excerpta Medica, North Holland, Amsterdam, Oxford, New York, 1978: 327-348.
Olton, D.S., Walker, J.A., Wolf, W.A. A disconnection analysis of hippocampal function. Brain Res., 1982, 233, 2: 241-253.
Oniani, T.N. Neurophysiological processes underlying the short-term memory in "pure form". In. T.N. Oniani (Ed.), Neurophysiological Aspects of Memory. Gagrskie Besedy, Metsniereba, Tbilisi, 1979, VII: 560-582 (in Russian).
Oniani, T.N. The Integrative Function of the Limbic System. Metsniereba, Tbilisi, 1980 (in Russian).
Oniani, T.N., Koridze, M.G., Kavkasidze, M.G., Gvetadze, L.B. The dynamics in excitability of various brain structures during different phases of wakefulness-sleep cycle. In: T.N. Oniani (Ed.), Neurophysiology of Emotion and Wakefulness-Sleep Cycle. Metsniereba, Tbilisi, 1974, I: 120-159 (in Russian).
Oniani, T.N., Mgaloblishvili, M.M., Keshelava, M.V. Activation and inactivation of conditioned foodmotor reflex by electrical stimuation of the subcortical structures and the dynamics of conditioned delayed reactions. In: T.N. Oniani (Ed.), Neurophysiology of Emotion and Wakefulness-Sleep Cycle. Metsniereba, Tbilisi, 1976, II: 117-134 (in Russian).
Oniani, T.N., Nachkebia, A.Y. On the problem of hippocampo-mesodiencephalon interrelations. Fiziologicheski Zhurnal, 1982, 28, 6: 684-693 (in Russian).
Oniani, T.N., Vartanova, N.G. Factors of extinction of alimentary instrumental conditioned reflex. Acta Neurobiol. Exp., 1980, 40: 173-198.
Ott, T. The role of hippocampal neurotransmitters in the generation of theta rhythm and memory storage. In: T.N. Oniani (Ed.), Neurophysiological Aspects of Memory. Gagrskie Besedy. Metsniereba, Tbilisi, 1979, VII: 546-560.
Penfield, W., Milner, B. Memory deficit produced by bilateral lesion in the hippocampal zone AMA. Arch. Neurol. Psychiatry, 1958, 79: 475-497.
Petsche, H., Stumpf, Ch. Topographic and toposcopic study of origin and spread of the regular synchronized arousal pattern in the rabbit. Electroenceph. Clin. Neurophysiol., 1960, 12: 589-600.
Petsche, H., Stumpf, Ch., Gogolak, G. The significance of the rabbit's septum as a relay station between the midbrain and hippocampus. Electroenceph. Clin. Neurophysiol., 1962, 14: 201-211.
Pickenhaim, L., K1ingberg, F. Hippocampal slaw wave activity as a correlate of basic mechanisms in the rat. In: Adey and Tokizane (Eds.), Progress in Brain Research., 1967, 27: 218-227.
Pubols, L.M. Changes in food motivated behavior of rats as a function of septal and amygdaloid lesions. Exp. Neurol., 1966, l5: 240-254.
Shinkman, R., Kaufman, K. Time course of retroactive effects of hippocampal stimulation on learning. Exptl. Neurol., 1972, 34: 476-483.
Shwartzbaum, I.S., Kellicut, M.H., Spieth, L.M., Thompson, G.D. Effects of septal lesions on response inhibition associated with food reinforced behavior. J. Comp. Physiol., 1964, 58: 217-224.
Sedetz, F.J., Matalka, E.S., Burnell, B.N. Septal ablation and the social behavior of the golden hamster. Physiol. Behav., 1970, 6: 79-88.
Soumireu-Mourat, B., Destrade, C., Cardo, B. Effects of seizure and sub-seizure posttrial hippocampal stimulation on appetitive-operant behavior in mice. Behav. Biol., 1975, 15: 303-316.
Stein, D.G., Chorover, S.L. Effects of posttrial electrical stimulation of hippocampus and caudate nucleus on maze learning in the rat. Physiol. Behav., 1968, 3: 787-791.
Vardaris, R.M., Schwartz, E.E. Retrograde amnesia for passive avoidance produced by stimulation of dorsal hippocampus. Physiol. Behav., 1971, 6: 131-135.
Vinogradova, O.S. Hippocampus and Memory. Nauka, Moscow, 1975 (in Russian).
Vinogradova, O.S., Braznik, E.S. Neuronal aspects of septo-hippocampal relation. In: Functions of the Septo-Hippocampal System. Ciba foundation symposium. Elsevier. Excerpta Medica. North Holland, Amsterdam, Oxford, New York, 1978, 58 (new series): 145-177.
Wetzel, W., Ott, T., Matthies, H. Posttraining hippocampal rhythmic slow activity (theta) elicited by septal stimulation improves memory consolidation in rats. Behav. Biol., 1977, 21: 32-40.
Figures
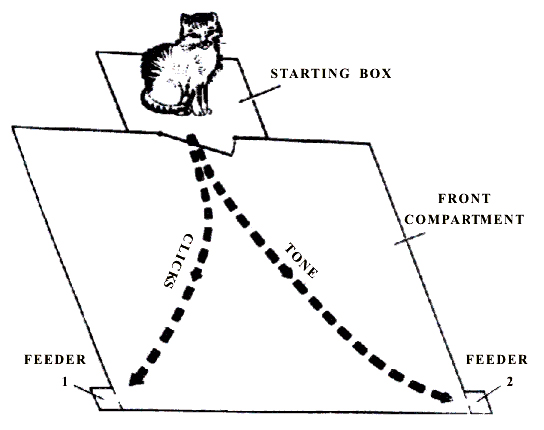
Figure 1. Floor plan of chamber for elaboration and extinction of instrumental alimentary behavior to two feeders.
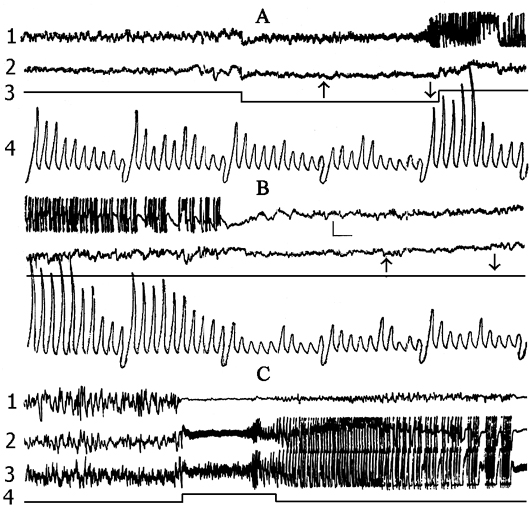
Figure 2. The local hippocampal epileptiform discharges elicited by electrical stimulation (5.5 V, 200 per sec, 0.1 msec) of its contralateral area.
Recordings in A and B: 1 - dorsal hippocampus, 2 - sensorimotor area of the neocortex, 3 - marking of electrical stimulation (deviation downward), 4 - integrated values of d (1-4 Hz), Q (4-7 Hz), a (8-12 Hz), b1 (12-20 Hz), b2 (20-30 Hz) rhythms within the dorsal hippocampus (the first 5 deviations) and sensorimotor cortex (the next 5 deviations). Integration epoch 5 sec. Calibration 200 mV, time 1 sec.
Recordings in C: 1 - sensorimotor cortex, 2 - contralateral ventral hippocampus, 3 - contralateral dorsal hippocampus, 4 - marking of electrical stimulation (5.5 V, 200 per sec., 0.1 msec). Stimulation switching is designated by upward rising line.
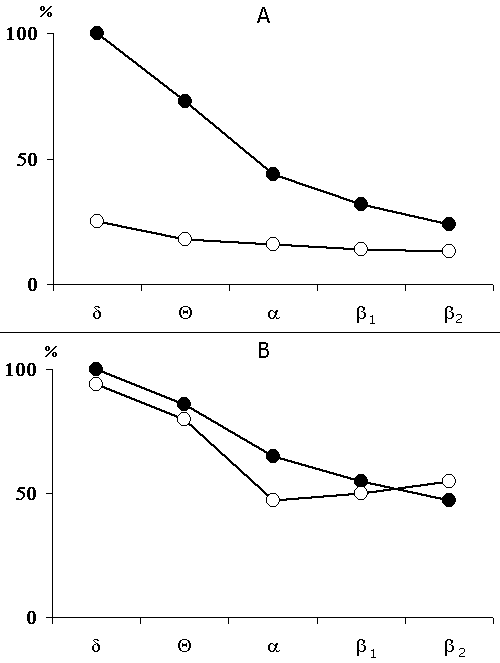
Figure 3. Change of the basic rhythms of the electrohippocampogram (A) and electrical activity from the sensorimotor area of the neocortex (B) as a result of repeated elicited (15 times) local epileptiform discharges in the hippocampus in response to electrical stimulation of the dorsal hippocampus.
Legends: black circles reflect data before the elicitation of epileptiform discharges, while light ones - following discharges.
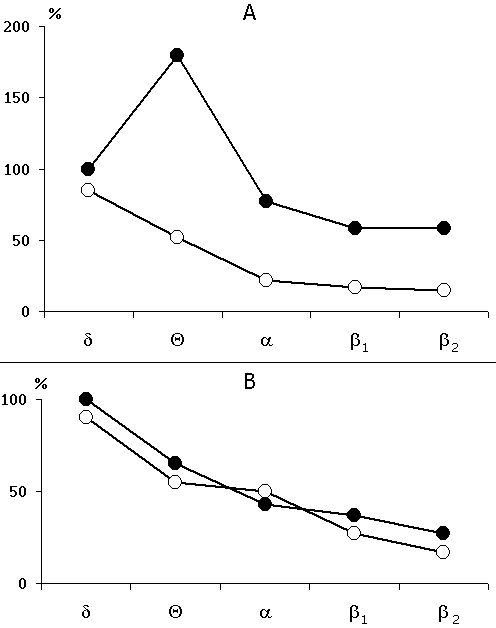
Figure 4. Change of the basic rhythms in the electrohippocampogram against the background of: A - paradoxical sleep and B - slow wave sleep, as a result of repeated (15 times) local epileptiform discharges, elicited by electrical stimulation of the dorsal hippocampus.
Legends: black circles reflect the rhythm before the elicitation of epileptiform discharges, while the light ones - following the discharges.
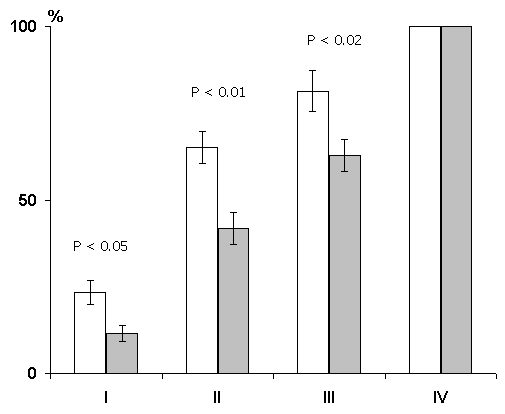
Figure 5. Influence of preliminary postconvulsive depression of the hippocampal theta rhythm on the elaboration of instrumental alimentary reflexes.
On the ordinate axis - percentage of correct responses; abscissa - learning sessions.
White columns in each pair - control group of animals; gray - experimental ones.
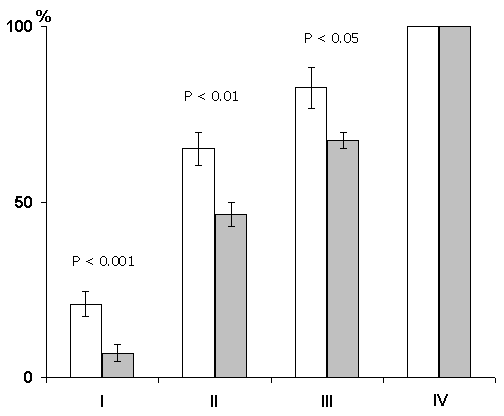
Figure 6. Influence of posttrial postconvulsive depression of the hippocampal theta rhythm on the elaboration of instrumental alimentary reflexes.
On the ordinate axis - percentage of correct responses; abscissa - learning sessions.
White columns in each pair - control group of animals; gray - experimental ones.
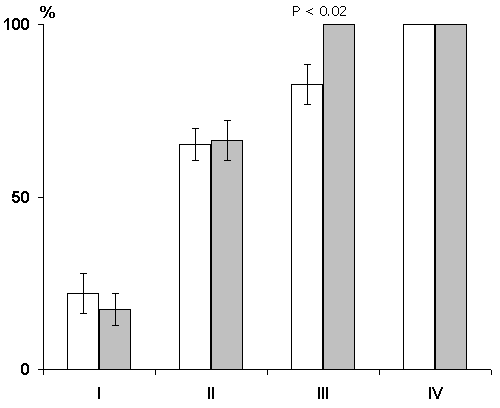
Figure 7. Influence of functional elimination of the hippocampus by local epileptiform discharges on the elaboration of instrumental alimentary reflexes.
Data obtained in experiments with induced local epileptiform discharges in the hippocampus 1 min after each reinforcement of conditioned stimulus with unconditioned.
On the ordinate axis - percentage of correct responses; abscissa - learning sessions. White columns in each pair - control group of animals; gray - experimental ones.
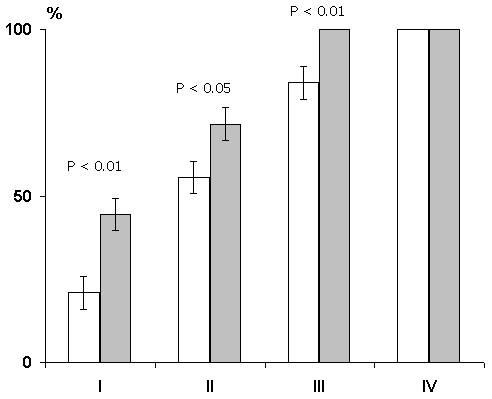
Figure 8. Influence of functional elimination of the hippocampus by local epileptiform discharges on the elaboration of instrumental alimentary reflexes.
Data obtained during induced local epileptiform discharges immediately after each reinforcement.
On the ordinate axis - percentage of correct responses; abscissa - learning sessions. White columns in each pair - control group of animals; gray columns - experimental ones.
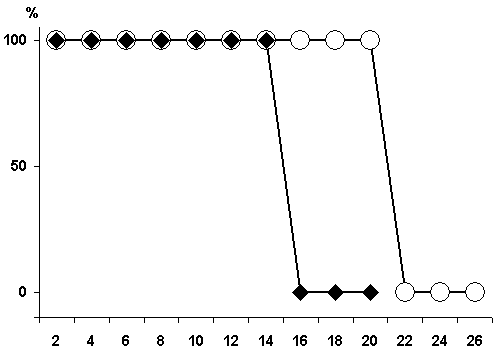
Figure 9. Influence of functional elimination of the hippocampus by local epileptiform discharges on acute extinction of conditioned instrumental alimentary reflexes.
On the ordinate axis - percentage of correct responses; abscissa - number of trials. White circle - control group of animals; black rhombus - experimental group of animals.
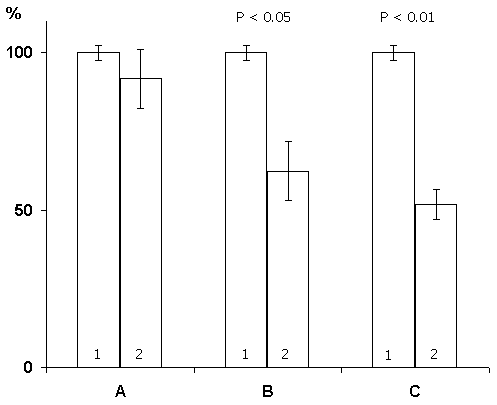
Figure 10. Influence of functional elimination of the hippocampus by local epileptiform discharges on the conditioned delayed reaction.
On the ordinate axis - percentage of correct reactions. Column 1 in each pairs presents data obtained in control group of animals. Column 2 - in experimental group of animals with the functional elimination of the hippocampus induced 10 (A), 5 (B), 2 (C) sec after the cessation of conditioned signal respectively.

Figure 11. The dynamics of electrical activity from the neocortex and hippocampus in the sleep-wakefulness cycle.
Recordings: 1 - sensorimotor area of the neocortex, 2 - dorsal hippocampus, 3 - electrooculogram. A - wakefulness, B - slow wave sleep, C - paradoxical sleep. Calibration 200 mV, time 1 sec.
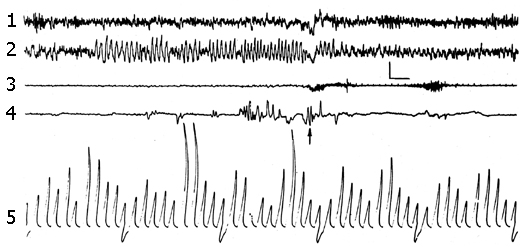
Figure 12.1. Change of electrical activity from the sensorimotor area of the neocortex and the dorsal hippocampus under the influence of nonemotional arousal of the animal from the paradoxical phase of sleep shortly after its onset.
Recordings: 1 - sensorimotor cortex, 2 - dorsal hippocampus, 3 - electromyogram (cervical muscle), 4 - electrooculogram, 5 - integrated values of d, q, a, b1, b2 rhythms within the sensorimotor cortex (the first five deviations) for the 5 sec epoch. The moment of awakening is indicated by an arrow. Calibration of intensity 200 mV, time 1 sec.
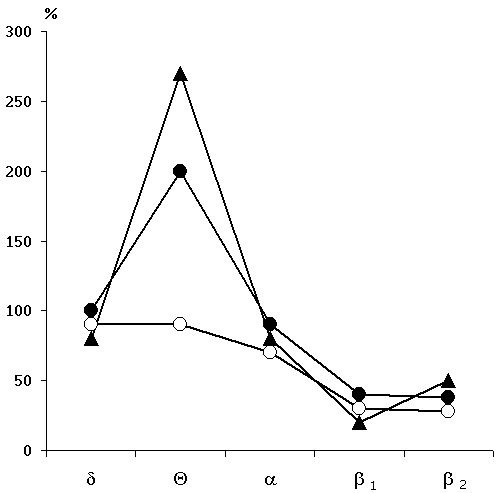
Figure 12.2. Expression of d, Q, a, b1, b2 rhythms composing the electrohippocampogram shown on the Figure 12.
Legends: black triangle - on the face of fully developed PS, black circle - at the onset of PS and white circle - following the nonemotional awakening from PS.
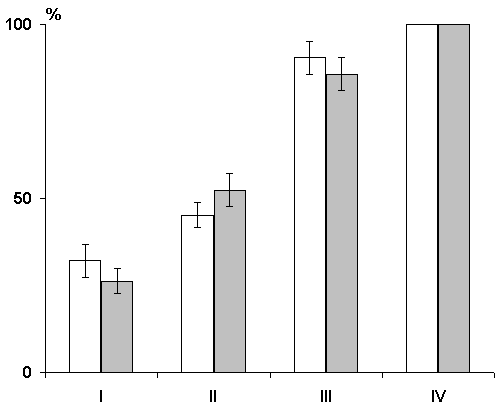
Figure 13. Influence of PSD by nonemotional awakening on the acquisition of instrumental alimentary reflexes.
On the ordinate axis - percentage of correct responses; abscissa - learning sessions. White columns in each pair - control group of animals; gray columns - experimental ones.
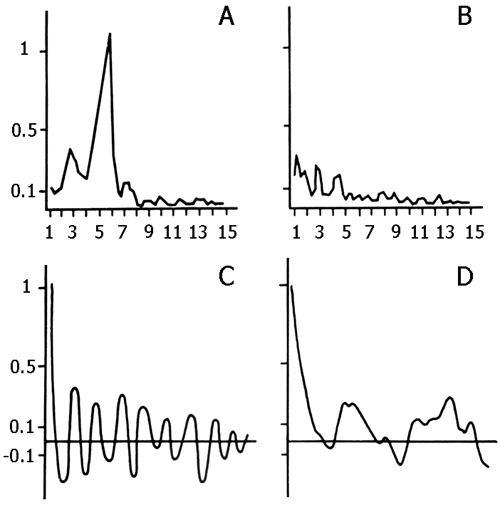
Figure 14.1. Spectral density and corresponding autocorrelograms of hippocampal electrical activity during active wakefulness.
A and C - baseline values; B and D - after lesion of medial septum. On the abscissa - the frequency in Hz, on the ordinate - relative amplitudes.
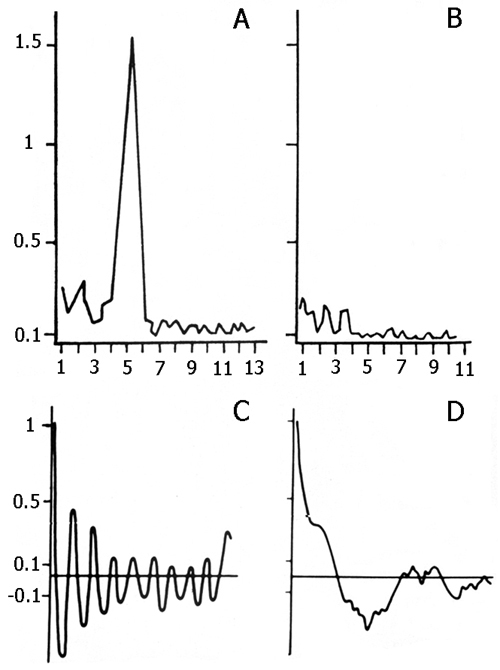
Figure 14.2. Spectral density and corresponding autocorrelograms of hippocampal electrical activity during paradoxical sleep.
A and C - baseline values; B and D - after lesion of medial septum. On the abscissa - the frequency in Hz, on the ordinate - relative amplitudes.
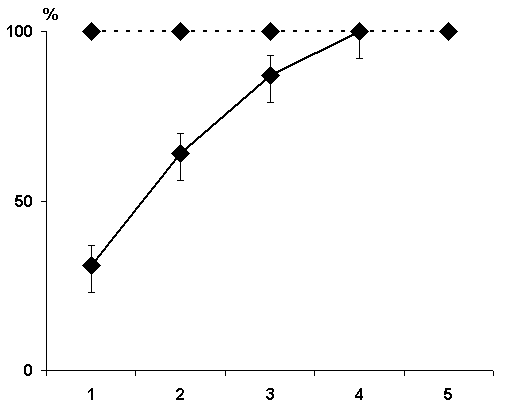
Figure 15. Influence of septal lesion on previously elaborated instrumental alimentary reflexes.
Dotted line: the data obtained after a medial septal lesion; solid line: total septal lesion. On the ordinate axis - percentage of correct responses, abscissa - experimental days.
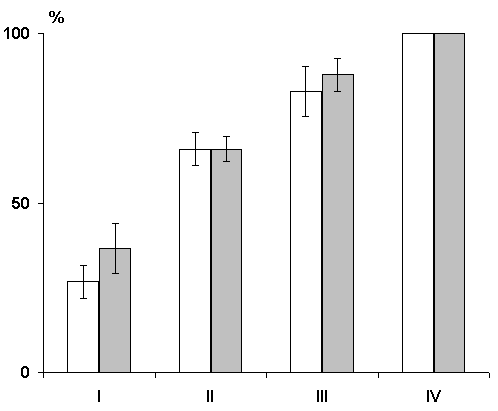
Figure 16. Influence of medial septal lesion on the elaboration of instrumental alimentary reflexes.
On the ordinate axis - percentage of correct responses; abscissa - learning sessions. White columns in each pair - control group of animals; gray columns - experimental ones.
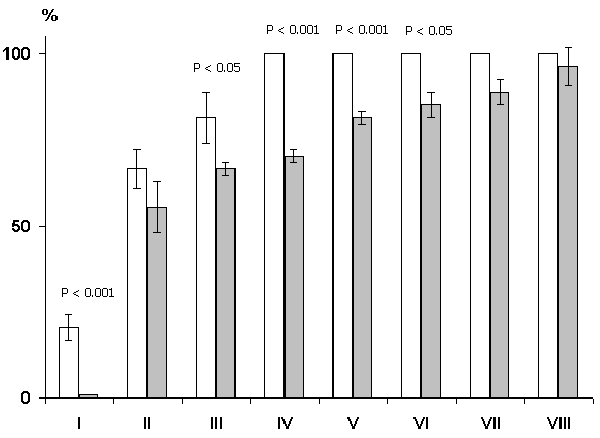
Figure 17. Influence of total septal lesion on the elaboration of instrumental alimentary reflexes.
On the ordinate axis - percentage of correct responses; abscissa - learning sessions. White columns in each pair - control group of animals; gray columns - experimental ones.
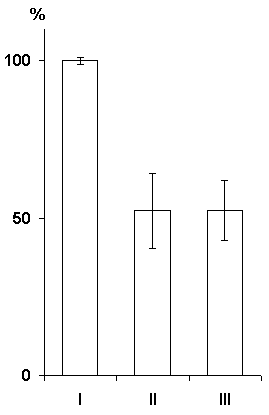
Figure 18. Influence of septal lesion on the performance of conditioned delayed reaction.
OThe first column presents the data in control animals, the second - the data obtained after lesion of the medial septum, the third - after total lesion of the septum. On the ordinate axis - percentage of correct responses.
Correspondence: Oniani Tengiz, Prof.,
Department of Neurobiology of Sleep-Wakefulness Cycle,
I.S. Beritashvili Institute of Physiology, Georgian Academy of Sciences,
14, L. Gotua str., Tbilisi, 380060, Georgia.
E-mail: nswc@neurobiology.ge



















