Neurobiology of Sleep-Wakefulness Cycle 1(1): 32 - 44, 2001
THE STRUCTURE OF THE SLEEP-WAKEFULNESS CYCLE DURING AND AFTER PARADOXICAL SLEEP DEPRIVATION BY THE WATER TANK METHOD
T. Oniani, I. Badridze, M. Mgaloblishvili-Nemsadze, N. Lortkipanidze, L. Maisuradze, E. Chidjavadze
Department of Neurobiology of Sleep-Wakefulness Cycle, I.S. Beritashvili Institute of Physiology, Georgian Academy of Sciences, Tbilisi
Accepted in revised form 12 October 2001; recieved 2 August 2001
Summary
The effect of 48, 72 and 92 hr water tank deprivation of paradoxical sleep (PSD) on the structure of the sleep-wakefulness cycle (SWC) was studied in cats with chronically implanted electrodes both in the deprivation and postdeprivation period.
Multiparametric analysis (observation on the dynamics of electroneocorticogram, electrohippocampogram, tonic activity of the skeletal muscles, electrocardiogram, eye movement, ponto-geniculo-occipital spikes, etc.) shows a strong stressful impact of the water tank situation on the experimental animal, on the face of which the development of the sleep cycle is being delayed. However, after the lapse of a definite time, in spite of the presence of a high emotional tension and inconvenient situation for taking an optimal position of the body to realize the cycle of sleep, the EEG parameters of the slow wave phase of sleep (SWS) (even at their most profound expression) develop rather intensively and last long enough to trigger the paradoxical phase of sleep (PS). As to the PSD in this situation, it is accomplished as behavioral awakening of the animal induced by the fear reaction associated with the development of the skeletal muscle atonia. Since similar awakenings are reiterated rather frequently, animals in the water tank are virtually in a permanent anxiety state even during deep SWS. The high level of emotional tension in the form of an after-effect persists in the animal for rather a long time in the postdeprivation period too. In spite of this it is namely at this time that the rebound of not only the deprived PS, but still more, of SWS is observed. On the basis of analysis of these facts it is concluded, that water tank method is not adequate for PSD. At the same time the character of the SWC course during the high emotional tension elicited by stressful factors is discussed. These data may also be beneficial while discussing the abundant controversial data accumulated in the literature concerning the effect of water tank PSD on the process of learning, as well as on the role of PS in the organization of memory in general.
Key Words: Sleep-wakefulness cycle, PS deprivation, SWS deprivation, water tank method, anxiety state.
Introduction
Since the discovery of the paradoxical phase of the sleep-wakefulness cycle (SWC) both in animals (Dement 1958; Jouvet et al. 1959) and man (Aserinsky and Kleitman 1953), the method of paradoxical sleep deprivation (PSD) (Dement 1960) has been widely employed on the one hand, for studying the neurobiological mechanisms of the so-called third state of the brain and its involvement in the integrative activity of the central nervous system (Jouvet 1962; Dement 1968; Oniani 1977) and on the other hand, it has been successfully applied in clinics for the purpose of treatment of some psychoneurologic diseases. In clinics the pharmacological method of PSD is mainly used (Vogel 1975; Vogel et al. 1988), while in animals preference is given to the non-pharmacological one (Dement et al. 1967; Jouvet 1967; Fergusson and Dement 1968; Fishbein and Gutwein 1977; Oniani 1984), the gist of which consists in ceasing the further development of paradoxical sleep (PS) immediately after appearance of the first signs of its onset in the SWC. Interruption of the normal development of PS is, as a rule, achieved by instantaneous awakening of the sleeping animal, after which, before the appearance of the next PS, it may sleep calmly. Since the realization of this method during continuous observation on the SWC is rather laborous, for the simplification of resolution of the task, more or less automatized methods of non-pharmacological PSD have been developed subsequently extensively applied (Jouvet 1965; Dement et al. 1967; Van Hulzen and Coenen 1980; Rechtschaffen et al. 1983). However, these methods for various reasons appeared to be inadequate, for they create rather acute conditions for the habitation of experimental animals, that, in its turn, stipulates development of stress. Amidst these stressing methods the most conventional is the so-called method of water tank, first developed by Jouvet for the experiments on cats (Jouvet et al. 1964), and Dement and Morden for the experiments on rats (Dement et al. 1967; Morden et al. 1967). The water tank method has been broadly applied and is being applied to the present time for the investigation of such fundamental functions and mechanisms of PS (see Jouvet 1972) as are the ways of formation of intrinsic need for it, the role of PS in the organization of long-term memory (Fishbein and Gutwein 1977; Bloch et al. 1981; Oniani 1984; Oniani and Lortkipanidze 1985), causal interrelation and interdependence of different SWC phases (Jouvet 1972; Kobayashi et al. 1985; Oniani et al. 1988a), etc. In spite of the fact that in the aspect of estimation of the given method its inadequacy for the proper study of namely the above-indicated neurobiological processes (Oniani 1984; Oniani and Lortkipanidze 1985), it is still commonly employed at the present time too (Sallanon et al. 1983; Camarini and Benedito 1997; Jouvet 1999; Sucheki et at. 1999; Mallick 2000). It should be also mentioned that while estimating the inadequacy of this method mainly those deviations from the norm were indicated which are observable in the postdeprivation period (Fishbein and Gutwein 1977; Oniani and Lortkipanidze 1985). Less attention was paid to studying the disturbances which, in addition to PSD, may develop in SWC during deprivation, although some efforts have been made in this direction too (Vimont-Vicary et al. 1966; Oniani et al. 1988b). The alteration of the structure of the SWC in the cat both during the water tank PSD and in the postdeprivation period was described in more or less details in the laboratory of M. Jouvet by Vimont-Vicary et al. (1966) as back as in 1966. On the basis of analysis of the data obtained a conclusion was drawn that the water tank deprivation technique seems to be selective in relation to PS. The question as to how much the given method meets the requirements of selective PSD, we shall try to discuss more comprehensively in the analysis of new facts. The inadequacy of the water tank technique in general has been indicated in our earlier communications (Oniani 1984; Oniani and Lortkipanidze 1985) in which the role of PS in the organization of learning and consolidation of memory traces was considered. In those experiments confirmation was found of the data by some authors (see Vogel 1975) indicating that the so-called PSD by this method causes nonspecific change of behavior of the experimental animal via the stressful influence of the water tank situation. The effects which are observed in the experimental animals during the application of learning and open field tests appeared to be the result of not selective PSD, but of the nonspecific increase of the brain excitability, hindering thereby its integrative activity in general.
In the experiments described below attention was concentrated on the analysis of those changes in SWC, which occur during PSD with the water tank method and which persist as after-effects in the postdeprivation period. For the more or less full estimation of the changes observable under the influence PSD with the water tank method, they were compared with those obtained in the same animals with the use of the so-called "classical" method (Dement 1960), i.e. when PSD is executed by the experimenter with a momentary non-emotional awakening of the animal right away after the appearance of each PS in SWC.
Methods
Experiments were conducted in 5 mature cats. For polygraphic recordings of SWC steel electrodes, with the uninsulated tip (150-200 m), were chronically implanted in different areas of the neocortex, dorsal hippocampus, oculomotor and cervical muscles and the lateral geniculate nucleus (LGN). The indifferent electrode prepared from silver wires was attached to the crest of the occipital area of the skull. To elicit awakening of the sleeping animal metallic bipolar electrodes were implanted also in the midbrain reticular formation and posterior hypothalamus. Coordinates for checking the localization of the electrodes' tips were derived from the atlas of Jasper and Ajmone-Marsan (1954). The subcortical activating structures were stimulated by rectangular impulses from the high-frequency output generator. Stimulation parameters could variate within wide ranges (0,5-20 V, 1-300 per sec, 0,1-5 msec).
The electrical activity from different areas (sensorimotor or visual) of the neocortex, dorsal hippocampus, LGN, oculo-motor and cervical muscles and electrocardiograms were monitored on a 13-channel ink-writing electroencephalograph of the Firm "San'ei". By means of a 2-channel analyser-integrator of the same Firm the amplitude-frequency analysis and integration of d (1-4 Hz), Q (4-8 Hz), a (8-12 Hz), b1 (12-20 Hz) and b2 (20-30 Hz) were made from one or another area of the neocortex and hippocampus. Recording of the integrated values of these rhythms was made consecutively, i.e. the rhythms were recorded first from the neocortex, then from the dorsal hippocampus. For statistical treatment of the rhythms their mean values were calculated for 2 min from the records of each phase of SWC. Duration of integration epoch was 5 sec.
PSD of various duration was made on one and the same cats using the water tank method, as well as nonemotional momentary arousal in the experimental chamber. In the case of application of the water tank method, pre- and postdeprivation recordings of SWC was carried out again in a comfortable experimental chamber with area 1m2 where animals had an access to food and water and possibility to sleep calmly. During application of this method, every day at 10.00 a.m. the cat was placed in the experimental chamber, was fed for 20-30 min. and again brought back to the water tank platform. For the purpose of comparison of PSD effects obtained with the use of water tank with the baseline values, continuous recording sessions of SWC had been run in all animals before they were placed on the platform.
Quantitative data were treated statistically and validity of the changes observed was checked by Student's t-criterion.
Results
1. SWC Dynamics before placement of experimental animals in the water tank.
Following post-operative recovery and a period of several days for habituation to the experimental chamber, a normal SWC was established in the cat. By this time the phase of wakefulness usually proceeds quietly, the cat either lying or sitting in high or low posture. If its eyes are normally open, then at the level of the neocortex desynchronization is usually observable (Fig. 1A). While in the hippocampus, depending on the wakefulness level, characteristic of this structure, theta rhythm is either feebly expressed or develops rather well (Fig. 1A). The cervical muscle, at this time, naturally shows good activity, the contraction of the oculo-motor one being but seldom and slow. Heart rate also depends on wakefulness level and is correlated with the expression of the hippocampal theta rhythm. So the level of the hippocampal theta rhythm and heart rate in a complex well reflect the dynamics of emotional tension at wakefulness.
The transition from passive wakefulness to slow-wave sleep (SWS), i.e. falling asleep in a comfortable experimental chamber takes place rather fast, but still gradually. At the beginning develops light SWS (LSWS), which is soon replaced by deep SWS (DSWS). Such transitions, under these conditions, are usually accomplished at the posture optimal for sleep. Very often the cat lies with the body stretched still at wakefulness and, respectively, occurs relaxation of the body. Very often passive wakefulness actually proceeds also in a sitting posture, but even in this case before the onset of LSWS the cat lies down and curls up. Naturally, the body gets more relaxed during DSWS (Fig. 1B) when tonic activity of the cervical muscles disappears altogether. At this moment electrical activity of both the neocortex and hippocampus shows the prevalence of waves of delta-, theta- and alpha range, while the beta rhythms appear less pronounced. During DSWS there is lack of considerable eye movements, the more so of rapid movements. Heart rate during DSWS is noticeably low as compared to wakefulness and what is more characteristic, it is even.
Under optimal conditions for a normal course of SWC, after a definite time there is, as a rule, a regular transition of DSWS to PS. This transition occurs faster than that of wakefulness to SWS, but still gradually (Moruzzi 1972; Oniani 1977; Oniani et al. 1978). The PS already fully formed principally differs not only from its preceding DSWS but also from wakefulness, although in the literature very frequently there is an indication that polygraphically this two phases of SWC are similar (Dement 1958; Jouvet 1962; Oniani 1980). As distinct from SWS, during PS in the electrical activity of the neocortex there occurs an acute suppression of slow rhythms (activation pattern), while in the hippocampus supperession of other slow rhythms is paralleled by a sharp increase of occurrence and regularity of the theta rhythm (Fig. 1C). Actually, the specific theta rhythm that is more or less characteristic for a waking state of the cat and its expression is dependent on the level of emotional tension, disappears in SWS, although the theta-constituents on the EEG of hippocampus are well pronounced thereat, they cannot be classified as the manifestation of theta rhythm. Except the complete skeletal muscle atonia, by which PS qualitatively differs not only from the phase of wakefulness, but also from DSWS (this is clearly seen in the cases when during DSWS the cervical muscle tone is not completely relaxed and on its face occurs the transition from SWS to PS), PS is specifically characterized by development of rapid eye movements, as well as by a sharp fluctuation of heart rate. Although in PS the EEG activation (desynchronisation) in the neocortex occurs so as is the case in active wakefulness, the two phases significantly differ from each other by this parameter too. In particular, in the cat's visual cortex (and not only in the visual) in PS develop frequent occipital spikes which are generated under the influences of pulses arriving from the pontine part of the midbrain (Jouvet et al., 1959) and extending via the lateral geniculate body over the cortex (see Jouvet 1972; Moruzzi 1972). Appearance of similar spikes in the neocortex during wakefulness may be viewed as a pathological disturbance of the SWC mechanisms, in particular as the development of hallucination processes (see Jouvet and Delorme 1965; Jouvet 1972). One can speak of similarity of wakefulness and PS in the aspect of dynamics of the organism's emotional tension although considering the expression and dynamics of hippocampal theta rhythm, as well as dynamics of heart rate, fluctuation of emotional tension in frequency and intensity, it is much higher in PS than in wakefulness with its various levels.
2. Cat's SWC in the water tank
As soon as the cat, already fit to have a normal SWC in a comfortable experimental chamber, for the purpose of PSD is placed on a restricted platform in the water tank, it falls into the state of anxiety, starts mewing and looks around with dilated pupils. Similar stress-induced state is clearly reflected in the SWC polygraphic records too. In this respect the most striking is the enhancement of hippocampal theta rhythm and acceleration of heart beats (Fig. 2A). The increase in both of the parameters appears statistically significant as compared even to active wakefulness in the SWC baseline records (Fig. 1A). At the same time, an obvious tension of somatic musculature is observable, from the cervical muscles on the face of expressive general tone one can register the high-amplitude spikes reflecting sporadic twitching of muscles, while the oculo-motor muscles contract (Fig. 1C) so as is typical for PS. The excited wakefulness of the experimental cats, placed in a water tank, usually lasts for rather a long time (about 6-8 hr), after which EEG may show the signs of LSWS, the course of which usually is not monotonous, but is again marked by occurrence of frequent arousals (Fig. 2C). During similar LSWS heart rate persists on a high level. Naturally, the high level of skeletal muscle tone is also maintained, as the cat in this state as well has to sit on the platform in the high sphinx posture. As been mentioned, LSWS is frequently interrupted by short-lasting arousals in view of inconvenient situation for the development of sleep. However, after such unexpected arousals rather long phases of wakefulness may develop. Mainly they proceed under high emotional tension that is accompanied by such EEG and somato-vegetative shifts as are seen in Fig. 2A, and seldom at a comparatively low level of emotional tension, also reflected in EEG and somato-vegetative parameters (Fig. 2B), these parameters are still higher that at active wakefulness in baseline records (Fig. 1A). Characteristically, at this time too, persist the signs of high somatic tension evidenced by separate sporadic twitching movements of the cervical muscles and the eye movement characteristic of PS.
After a definite time of being in the water tank in view of an increased need for sleep the cat takes the position on the platform as convenient as possible, is in the posture of a low sphinx, in the main, and seen in EEG is a clear pattern of DSWS (Fig. 3). At this time both in the neocortex and hippocampus dominate delta-, theta- and alpha-constituents of EEG. Naturally, the skeletal muscle tone is being maintained on a very high level, although it is not as pronounced as is observed during LSWS (Fig. 2C). Against the background of such electroencephalographic DSWS (EEG DSWS) rapid eye movements disappear and only the low-amplitude movements persist. As regards the heart rate, it is considerably higher in the EEG DSWS in the water tank than in the baseline records made in a normal experimental chamber (Fig. 1B). Statistical treatment of integrated rhythms indicates that the availability of a certain emotional tension in the EEG DSWS under conditions of a water tank, however, has an impact on its expression both in the neocortex (Fig. 4B) and hippocampus (Fig. 4A), compared to the baseline level. At the level of neocortex it is displayed in the authentic decrease of the amplitude of theta and alpha rhythms and augmentatation of beta rhythms. It is noteworthy that there is no marked change of the delta rhythm. These changes in both cases can be explained by the fact that under conditions of a water tank the EEG DSWS, however, occurs under a certain emotional tension, that, in its turn, is due to stressful factors in the water tank eliciting fear reaction and making it impossible to realize normal behavioral sleep, notwithstanding the development, as there were, normal EEG DSWS.
As indicated above, under conditions of a water tank, for some time after the cat had been placed on the platform, there is a total deprivation of sleep by means of its replacement by active wakefulness. Then develops LSWS during which frequent arousals are observable. After some time there also develop the fragments of the EEG DSWS, amount of which during the first 24-hr of the animal's presence in the water tank is rather modest (Fig. 5A, column 2). Then the amount of EEG DSWS progressively increases (Fig. 5A, column 3) and on the third day (Fig. 5A, column 4) reaches the baseline level (Fig. 5A, column 1). Just by this time one can see a substantial deceleration of heart rate, compared to the data of the first two days of the animal's being in the water tank, although it tangibly exceeds the baseline level (Fig. 6).
In spite of the fact the EEG DSWS in the water tank proceeds in the inadequate conditions for the proper realization of this physiological state of the brain, after a certain time it is, however, capable of triggering the work of neurophysiological mechanisms for PS. Yet, in view of development thereat of entire skeletal muscle atonia, PS cannot further develop, as the cat momentarily awakes to avoid falling from the platform into the water. Actually occurs self-deprivation of PS, what is just the aim of application of this method. The pattern EEG DSWS passing into PS and the following PS self-deprivation in the water tank looks peculiarly and differ from the pattern observable in the normal course of SWC. As mentioned above, the EEG DSWS under conditions of a water tank occurs on the face of expressed skeletal muscle tone that is necessary for the maintenance of a sitting posture of the body on the platform. In these conditions the start and transition of EEG DSWS to PS also occurs with the pronounced skeletal muscle tone (Fig. 7A). The first signs of skeletal muscle atonia are, however, observable shortly after the onset of EEG desynchronisation, characteristic of the transient phase from SWS to PS in the normal course of SWC, but soon the tone recovers, irrespective of the persistence of EEG signs of the transient phase (Fig. 7A). It is followed by a more clear-cut atonia which starts but after appearance of eye movement and enhancement of hippocampal theta rhythm (Fig. 7B). Here the mechanisms of PS self-deprivation is being triggered and the cat wakes up (Oniani et al. 1988b). In accordance with the dynamics of the skeletal muscle tone at the transition from DSWS to PS, in this case too, there is a consistent heart rate variation as is the case in the normal course of SWC. With the appearance of signs of the skeletal muscle atonia heart rate decelerates and then after the onset of self-deprivation, it recovers to the level characteristic of the waking state under conditions of water tank. Thus, the prerequisite for self-deprivation in the water tank is the development of fear reaction. Thereby every time occurs withdrawal of those adaptive processes which have anyway developed in SWS, and restoration of emotional tension under the impact of stressful factors of the set. In view of this the emotional tension at wakefulness in the water tank persists on a high level and there occurs no marked reduction of it even for 3 days. This is well testified, apart from behavioral reactions and EEG indices, by the heart rate dynamics as well (Fig. 6). The heart rate in the water tank during wakefulness (Fig. 6A) seems not to decrease markedly even on the third day. Although during SWS the heart rate, as compared to wakefulness, is considerably decreased even in the water tank, but it is higher than the baseline level (Fig. 6B). Characteristically, this level during SWS too anyway persists during three days of the animal's stay under conditions of the water tank.
That the reason for the elevated emotional tension, and especially, its further persistence in the water tank is not direct PS deprivation, but the impact of stressful factors elicited by the conditions of the water tank, are clearly indicated by the data obtained in SWC recordings again in the similar water tank but with larger diameter (16,5 cm) of the platform. After a certain adaptation period to the conditions of the water tank the cat has the possibility to take the posture at which it can realize the entire SWC. At this time DSWS usually develops in the absence of the cervical muscle tone perhaps due to the convenient mechanical position of the head (Fig. 8A). However, cardiac rhythm during DSWS in the water tank, in this case too, is considerably higher than in the baseline records. During transition from DSWS to PS at the beginning heart rate markedly decelerates, perhaps, again because enhancing atonia, but then with the development of normal PS (Fig. 8B), it starts fluctuating within wide ranges, that is characteristic of the present state. However as in PS in the water tank total frequency of cardiac rhythm is higher than in baseline records under condition of experimental chamber. This, together with the noticeably accelerated eye movements, as well as expression of hippocampal theta rhythm, indicate that the traces of stressing impact of the water tank on the animal's organism persist also in PS during its realization under conditions of a water tank.
Thus, the existence of high emotional tension, elicited by water tank, although in this case too delays the onset of proper SWC, but afterall it is realized. This means that the reason for PSD is not the high emotional tension observed in the cat under water tank conditions, but the impossibility for the skeletal muscles to develop atonia that is necessary for PS development.
3. Postdeprivation changes in the SWC structure
Following a 3-day long stay in the water tank where full PSD is achieved, the cat placed in the experimental chamber soon falls asleep and manifests normal SWC. However, by the phase ratio, the postdeprivation SWC significantly differs from that observed in the predeprivation records. This is primarily expressed in an increase of amount of both DSWS and PS (Fig. 5B, column 1). During the first 24-hr more significant is the rebound of PS than of SWS, later on during the next 48-hr, in parallel with an increase in the amount of SWS, that of PS gradually decreases, but especially sharply decreases the amount of wakefulness (Fig. 5B, columns 2, 3). After this starts to decrease also SWS amount and on the fifth day (Fig. 5B, column 4) the phase ratio in SWC recovers to the baseline level (Fig. 5A, column 1). Principally the same picture is observed in SWC phase ratio also after the cat's 48-hr stay in the water tank with the platform, on which the transition from SWS to PS becomes impossible (Fig. 9A). In this case too during the first recovery day the both sleep phases increase sharply, i.e. occurs a rebound not only of PS, but also of SWS (Fig. 9A, column 2). On the second postdeprivation day there is a marked decrease in PS amount (though it still tangibly exceeds PS amount in baseline records) and there is also noticeable, but still increase of SWS (Fig. 9A, column 3), compared to the first day. During the third (Fig. 9A, column 4) postdeprivation day a considerable parallel decrease is already observed in the amount of both sleep phases (but again they both exceed the levels in baseline records) and the normal structure of SWC recovers only on the fourth day (Fig. 9A, column 5).
As to the cardiac rhythm, for rather a long time (about 30-40 hr) it remains considerably accelerated in comparison with the baseline level even following PSD by the water tank method and when the cat is placed in the chamber comfortable for SWC realization (Fig. 10). At the same time, apparently in this situation too a considerable acceleration of cardiac rhythm is observed not only at wakefulness, but also in both phases of sleep. Recovery of the normal cardiac rhythm occurs only by the third or fourth day.
Apart from the postdeprivation increase in the total amount of SWS and PS, they enchance also in the intensity. Fig. 11A displays the comparison of pronounceness of delta, theta, alpha, b1 and b2 rhythms (or rather EEG constituents) observed in baseline SWC and in SWS following PSD by the water tank method. As is seen, all rhythms in a sensorimotor area of the neocortex in the postdeprivation SWC exceed in the amplitude the respective rhythms in baseline records. In addition to augmentation of the slow EEG constituents, attention attracts a reliable increase of comparatively high-frequency beta rhythms (especially b2), indicating relatively high activity of the brain, irrespective of deepening at this time of the so-called "delta sleep".
As to how the PS intensity changes in the postdeprivation period can be judged by comparison of the expression of hippocampal rhythms with the same rhythms in baseline records. As seen in Fig. 11B, during the first postdeprivation day, compared to the baseline SWC, there is a particularly strong enhancement of theta and suppression of alpha rhythm. Both of these facts imply the occurrence of elevated emotional tension in postdeprivation PS, compared to baseline level.
4. Effect of PSD by the instantaneous awaking method on the structure of SWC during deprivation and postdeprivation period
To elucidate the meaning of the impact of stressful factors, attending the PSD procedure by water tank method, on the SWC structure during deprivation, as well as in the postdeprivation period, we thought it expedient to carry out in the same cats experiments using PSD method by instantaneus awakening in the chamber comfortable for SWC realization. PSD experiments in this case were started after the animals had been fully habituated to the chamber conditions for the optimal realization of the entire SWC. Awakening of the animal was achieved by the threshold stimulation of either the posterior hypothalamus, or the midbrain activating reticular formation immediately after the onset of the basic parameters (desynchronized electrical activity in the neocortex, appearance of hippocampal theta rhythm, an increase in the PGO spikes, which appear still in SWS in a single form, rapid eye movement, etc.), characteristic of the normal PS onset. As is well known, with the use of this PSD method, progressively increasing PS onsets are observable and, accordingly a progressive increase in frequency of stimulation is necessary for their cessation (Fig. 12). A similar procedure for 48 hr virtually does not alter total SWS amount during deprivation (Fig. 9B, column 2 and 3), but the amount of wakefulness increases substantially, perhaps, at the expense of lack of PS. In the postdeprivation period too, during the first days (Fig. 9B, column 4), no marked changes are observable in SWS, compared to the baseline level (Fig. 9B, column 1), but there is an obvious PS rebound (Fig. 9B, column 4). On the second postdeprivation day (Fig. 9B, column 5) the ratio of different SWC phases returns to the level observable in baseline records.
In contrast to the data obtained with the application of the water tank method, at PSD by way of nonemotional awakening no marked changes are observed in the cardiac rhythm at wakefulness and in SWS during and after deprivation (Fig. 13A, B). As regards PS, a noticeable increase in heart rate is observed only during rebound of this phase (Fig. 13C). During PS rebound there is a significant increase also in the frequency of PGO spikes, eye movement and hippocampal theta rhythm. As to the intensity of SWS, by expression of the basic EEG - constituent rhythms, it does not differ from the baseline SWS either during PSD, or during postdeprivation PS rebound.
The facts mentioned above speak in favour of the PSD via instantaneous awakening not being a stressogenic each time of PS onset and therefore, does not alter tangibly the EEG and somato-vegetative parameters of SWC either during deprivation or postdeprivation PS rebound.
Discussion
Analysis of the material described above enables first of all to substantiate that application of the water tank method for PSD affects not only PS, but also all other SWC phases and stages because of a dramatic elevation in this situation of emotional tension in experimental animals. The stressing effect of the situation in the water tank manifests itself especially well at wakefulness, that finds vivid reflection in behavioral, EEG and somato-vegetative parameters of this state. In addition to such behavioral manifestations of fear reaction as mewing, dilatation of pupils and other signs, strong elevation of emotional tension is also testified by EEG changes (in particular, a sharp increase of hippocampal theta rhythm) and somato-vegetative parameters (e.g. acceleration of cardiac rhythm and the skeletal muscle high tone). All this results not only in PSD, that is the aim of application of this method, but also in a tangible delay of SWC realization in general. Therefore, during 12 hr, on an average, after the cat had been placed in the water tank, total deprivation of sleep is observable and, what is especially important to mention, not by means of mere nonemotional wakefulness, but by means of highly emotional wakefulness. The level of high emotional tension is observed also after the SWC recovery in the water tank as the development of more or less prolonged fragments of SWS. In this context it is especially noteworthy that high emotional tension persists in experimental animals also after the recovery in the water tank, as it were, of proper DSWS phases (Fig. 3). This is indicated primarily by the dynamics of somato-vegetative components and EEG DSWS. Besides, at every interruption of EEG DSWS by its transition to wakefulness, that is not infrequently observed in this situation, emotional tension by all parameters rises still higher. Similar acute shifts in emotional tension are seen at the cessation of started PS, i.e. at its deprivation with the water tank method. The thing is that during application of this method interruption of the started PS occurs by the animal's awakening under the effect of fear, elicited by expectancy to fall into the water. It is clear that this moment is the utmost stressing factor during the entire PSD procedure in the water tank. An acute elevation at this time of the animal's emotional tension is indicated both by EEG (augmentation of hippocampal theta rhythm), and somato-vegetative parameters (acute acceleration of cardiac rhythm and twitching movement of the cervical muscles with subsequent maintenance of high level of their tone) (Fig. 2A). It is clear that a progressive enfrequentation of these events, characterizing PSD in this situation too, is of course, promoted not only by maintenance of general background of elevated emotional tension throughout the deprivation period. This implies that the water tank situation appears to be inadequate not only for the normal course of other, supposed not deprived phases (wakefulness and SWS) of SWC, but also for PSD. It is naturally expected that all this can affect also the SWC structure in the postdeprivation period.
Continuous SWC recordings for several postdeprivation days in the experimental chamber, i.e. in the conditions comfortable for the realization of all its phases have detected considerable changes not only in PS, but also in other phases of the cycle. In parallel to the PS rebound there is no less intensive DSWS rebound too, irrespective of the fact that its total amount during deprivation is, as it were, unaltered in comparison with the baseline level (Fig. 5A). The fact also is striking that during the first postdeprivation day when there is a maximal PS rebound the DSWS rebound is less pronounced than in the subsequent two days (Fig. 5B), when PS amount gradually decreases and approaches the level, characteristic of the baseline SWC. Regular dynamics, in the postdeprivation period, is undergone also by cardiac rhythm. The accelerated heart rate observable in the water tank situation returns to the baseline level in the postdeprivation period, so that DSWC rebound in the first three postdeprivation days proceeds on the face of still tangibly accelerated cardiac rhythm (Fig. 10). With the recovery of normal ratio of SWC phases in the postdeprivation period cardiac rhythm also resumes the baseline values.
That the above-described changes in the SWC structure, observable in the recovery period, are stipulated by continuous elevation of emotional tension, as well as by other factors acting while using the PSD water tank method, is indicated by comparison of this data with those obtained with the application of nonstressful PSD method. As been demonstrated above, in the case of use for PSD of nonemotional awakening of the animal immediately after the appearance of signs of passing SWS into PS, in the postdeprivation period rebound of only deprived phase, i.e. PS, is observed, while SWS amount does not alter. Characteristically, the PS rebound does not last so long as is the case with the application of the water tank method and in compliance with it the normal SWC recovers faster. The lack of significant alteration of emotional tension while using this method of PSD is indicated by the lack of changes in cardiac rhythm (Fig. 13) and other indices of emotional state. The fact that in the recovery period during PS rebound cardiac rhythm appears to be noticeably greater than in baseline records is a consistent reflection of the pressure of PS need accumulated during PSD.
In summary, it may be said that the water tank method is inadequate for the achievement of selective PSD or for the elucidation of the role of PS in the brain integrative activity. However, the described data, except the estimation of the water tank method for selective PSD, supply important information as to how the SWC may generally proceed in the conditions of availability of the stress eliciting factors and as to how prolonged the after-effect may be after their abolishment.
It seems that the findings described in the present study will arouse interest of the investigators concerned with the SWC neurobiology and will be taken into account during further use of the water tank method and other stress techniques (Rechtschaffen et al. 1983) while studying the role of PS in memory organization, as well as the neurochemical processes that underlie the formation of intrinsic need for sleep in general and for its phases in particular.
References
Aserinsky, E., Kleitman, N. Regulary occurring periods of eye motility and concomitant phenomena during sleep. Science, 1953, 118, 3062: 273-274.
Bloch, V., Hennevin, E., Leconte, P. The phenomenon of paradoxical sleep augmentation after learning. Experimental studies of its characteristics and significance. In: W. Fishbein (Ed) Sleep, Dreams and Memory. N.Y. MTP. Acad. Press, 1981: 1-18.
Camarini, R., Benedito, M. Rapid eye movement (REM) sleep deprivation reduces rat frontal cortex acetylcholinesterase activity. Braz. J. Med. Biol. Res., 1997, 30, 5: 641-647.
Dement, W. The occurrence of low voltage, fast electroencephalogram patterns during behavioral sleep in the cat. Electroenceph. clin. neurophysiol., 1958, 10, 2: 291-296.
Dement, W. The effect of dream deprivation. Science, 1960, 131: 1705-1707.
Dement, W. The biological role of REM sleep. Circa 1968. In: A. Kales (Ed) Sleep physiology and Pathology. Philadelphia, Lippincott, 1969: 245-265.
Dement, W., Henry, P., Cohen, H., Fergusson, J. Studies on the effect of REM deprivation in humans and animals. In: S. Kety, E. Evarts and H. Williams (Eds) Sleep and Altered States of Consciousness. Williams and Wilkins, Baltimore, Maryland, 1967: 456-468.
Fergusson, J., Dement, W. Changes in intensity of REM sleep with deprivation. Psychophysiology, Baltimore, 1968, 4, 4: 300-312.
Fishbein, W., Gutwein, B. Paradoxical sleep and memory storage processes. Behav. Biol., 1977, 19, 5144: 425-464.
Jasper, H., Ajmone-Marsan, C. A stereotaxic atlas of the diencephalons of the cat. The National Research Council of Canada, 1954.
Jouvet, D., Vimont, P., Delorme, F., Jouvet, M. Etude de la privation selective de la phase paradoxale de sommeil le chat. C. R. Soc. Biol., Paris, 1964, 158, 4: 756-759.
Jouvet, M. Recherches sur les structures nerveausis et les mechanisms responsables des differences phases du sommeil physiologique. Arch. Ital. Biol., 1962, 100, 2: 125-206.
Jouvet, M. Behavioral and EEG effect of paradoxical sleep deprivation in the cat. Proc. 23rd Int. Congr. Physiolog. Sci., Tokyo, 1965, 4, 87: 344-355.
Jouvet, M. The neurophysiology of the states of sleep. Physiol. Rev., 1967, 47, 2: 117-177.
Jouvet, M. The role of monoamines and acetylcholine containing neurons in the regulation of the sleep-waking cycle. Ergebn. Physiol., 1972, 64: 166-307.
Jouvet, M. The Paradox of Sleep. (English) A Bradford book, 1999.
Jouvet, M., Delorme, F. Locus coeruleus et sommeil paradoxical sleep. C. R. Soc. Biol., 1965, 159, 4: 895-899.
Jouvet, M., Michel, F., Courjon, I., Hermann, H. Lactive electrique du rhinencepale au cours du sommeil chet le chat. C. R. Sec. Biol., Paris, 1959, 153, 1: 101-105.
Kobayashi, T., Tsuji, Y., Eudo, S. Sleep cycle as a basic unit of sleep. In: H. Schulz and P. Lavie (Eds) Ultradian Rhytms in Physiology and Behavior. Springer-Verlag Berlin Heidelberg, 1985: 260-269.
Mallick, B. One of the functions of REM sleep is to maintain brain excitability possible cellular mechanisms of action. Proc. of the 3rd Congress of ASRS in Thailand, Bangkok, 2000: A32.
Morden, B., Mitchell, G., Dement, W. Selective REM sleep deprivation and compensation phenomena in the rat. Brain Res., 1967, 5, 3: 339-349.
Moruzzi, G. The sleep-waking cycle. Ergebn. Physiol., 1972, 64, 1: 64-165.
Oniani, T. On the functional significance of sleep. Acta Neurobiol. Exp., 1977, 37, 4: 223-246.
Oniani, T. The Integrative Function of the Limbic System. Metsniereba, Tbilisi, 1980, (in Russian).
Oniani, T. Does paradoxical sleep deprivation disturb memory trace consolidation? Physiol. Behav., 1984, 33, 5: 687-692.
Oniani, T., Lortkipanidze, N. Effect of paradoxical sleep deprivation on learning and memory. In: T. Oniani (Ed) Neurophysiology of Motivation, Memory and Sleep-Wakefulness Cycle. Metsniereba, Tbilisi, 1985, 4: 136-214.
Oniani, T., Lortkipanidze, N., Mgaloblishvili, M., Maisuradze, L., Oniani, L., Babilodze, M., Gvasalia, M. Neurophysiological analysis of paradoxical sleep deprivation. In: T. Oniani (Ed), Neurobiology of Sleep-Wakefulness Cycle. Metsniereba, Tbilisi, 1988a: 19-43.
Oniani, T., Maisuradze, L., Lortkipanidze, N., Oniani, L. Paradoxical sleep self-deprivation in cats. J. VND, 1988b, 38, 2: 266-274, (in Russian).
Oniani, T., Molnar, P., Naneishvili, T., The nature of paradoxical phase of sleep. Neuroscience Translation. Fed. Amer. Soc. Exp. Biol., 1978, 15, 1.
Rechtschaffen, A., Gilliand, M., Bergmann, B., Winter, J. Physiological correlates of prolonged sleep deprivation in rats. Science, 1983, 221, 4606: 182-184.
Sallanon, M., Sanin, M., Buda, C., Jouvet, M. Serotoninergic mechanism and sleep rebound. Brain Res., 1983, 268: 95-104.
Sucheki, D., Duarte Palma, B., Tufik, S. Sleep rebound in rats following sleep deprivation induced by the modified multiple platform method. Sleep Research Online, Dresden, Germany, 1999, 2 (suppl. I): 559.
Van Hulzen, Z., Coenen, A. The pendulum technique for paradoxical sleep deprivation in rats. Physiol. Behav., 1980, 25, 5: 807-811.
Vimont-Vicary, P., Jouvet-Mounier, D., Delorme, F. Effects EEG et comportementaux des privations de sommeil paradoxal chez le chat. Neurphysiol., 1966, 20: 439-449.
Vogel, G. A review of REM sleep deprivation. Arch. Gen. Psychiat., 1975, 32, 6: 749-761.
Vogel, G., Roth, T., Gillin, J., Mendelson, W., Buffenstein, A. REM sleep and depression. In: T. Oniani (Ed) Neurobiology of Sleep-Wakefulness Cycle. Metsniereba, Tbilisi, 1988: 187-215.
Acknowledgments
This research was supported by the Japanese Government, under the ISTC grant G-391.
Figures
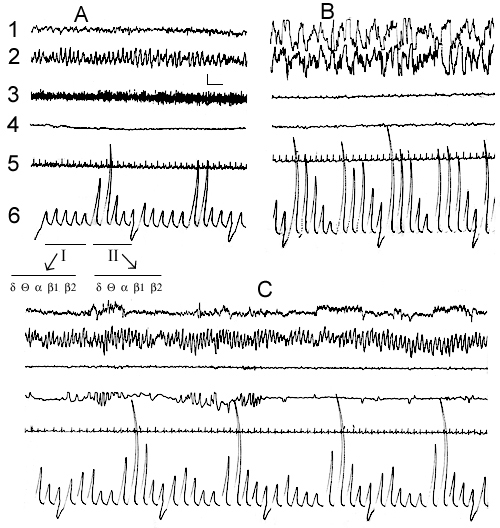
Figure 1. Electrical activity of the neo- and archipaleocortex, muscle tone, eye movements and cardiac rhythm at active wakefulness (A), DSWS (B) and PS (C) during SWC recording in the comfortable experimental chamber.
Leads: 1, sensorimotor area of the neocortex; 2, dorsal hippocampus; 3, cervical muscle; 4, oculomotor muscle; 5, cardiac rhythm; 6,
the integrated values of d, Q, a, b1 and b2 rhythms from the sensorimotor area of the neocortex (first five deviations I) and dorsal hippocampus (subseqent five deviations II); Duration of integration epoch-5sec;
Calibration: 200mV, 1sec.
Leads, calibration and notation on this and figures 2, 3, 7 and 8 are the same.
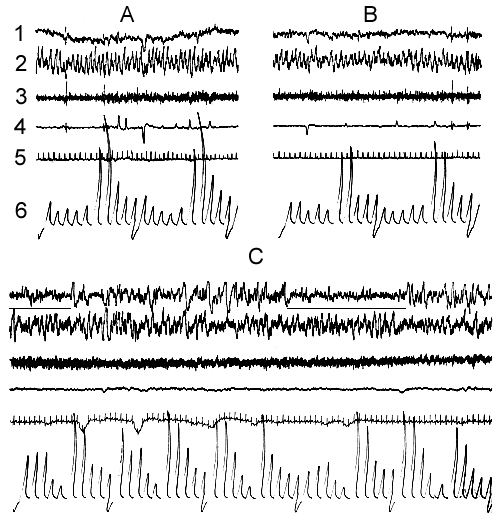
Figure 2. Electrical activity of the neo- and archipaleocortex, muscle tone, eye movements and cardiac rhythm under conditions of a water tank.
A, emotional wakefulness; B, active wakefulness; C, LSWS. The horizontal lines under the electroneocorticogram mark the moments of spontaneous awakenings.
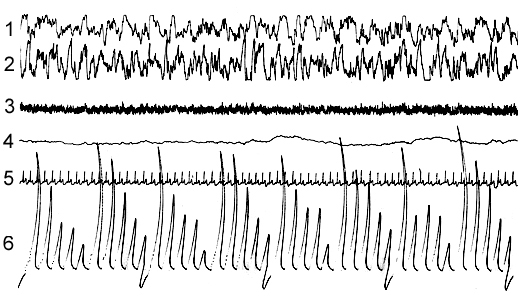
Figure 3. Electrical activity of the neo- and archipaleocortex, muscle tone, eye movements and cardiac rhythm during EEG DSWS under conditions of water tank.
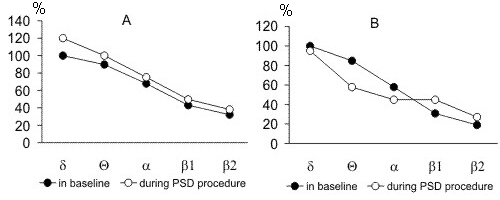
Figure 4. The pronounceness in the amplitudes of d, Q, a, b1 and b2 rhythms from hippocampus (A) and sensorimotor area of the neocortex (B) at SWS in the baseline recordings and recordings of deprivation procedure by water tank method. (the amplitude of baseline d rhythm is taken to be 100%).
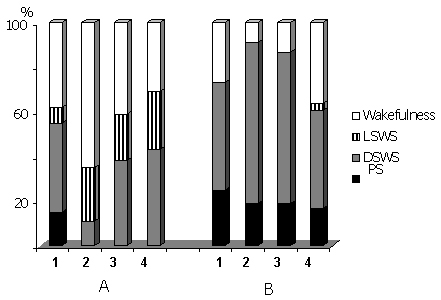
Figure 5. Percentage ratio of various phases of the SWC in the time course of 72 hr water tank PSD (A) and after cessation of deprivation procedure in the postdeprivation period (B).
Columns on A: 1-baseline, 2-first, 3-second and 4-third days in the water tank. Columns on B: 1-first, 2-second, 3-third and 4-fifth postdeprivation days.
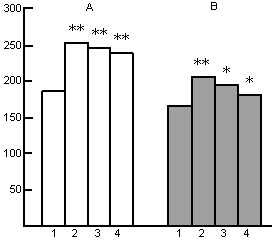
Figure 6. Influence of water tank PSD on the heart rate at wakefulness (A) and SWS (B) during deprivation procedure.
On the ordinate: frequency of heart beats;
Columns: 1-baseline, 2-, 3- and 4- first, second and third days respectively in the water tank;
(*p<0.02; **p<0.01; compared with baseline).
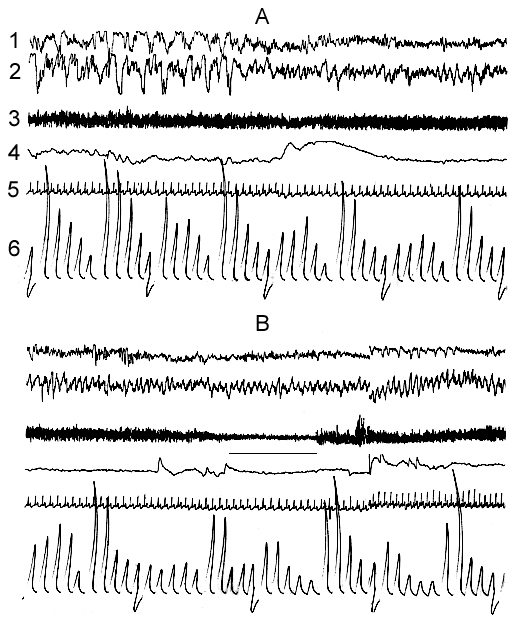
Figure 7. Dynamics of electrical activity of sensorimotor area of the neocortex and hippocampus, and somato-vegetative components at A, the transition from SWS to PS; B, its start and self-deprivation under the water tank conditions. (The cat is placed on the platform with 12cm diameter).
The horizontal line under electromyogram marks the moment of sceletal muscle atonia.
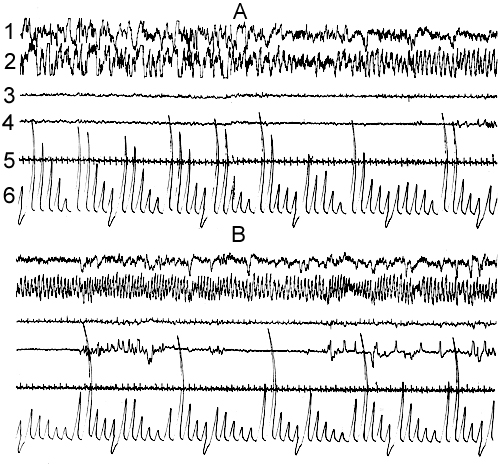
Figure 8. Dynamics of electrical activity of sensorimotor area of the neocortex and hippocampus and somato-vegetative components at A, the transition from SWS to PS and B, PS occurence under conditions of water tank by the platform with 16,5cm diameter.
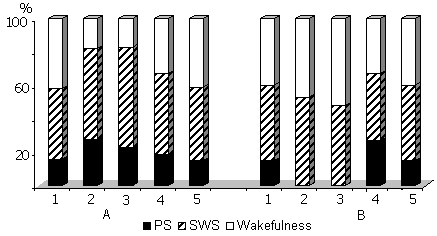
Figure 9. Influence of 48 hr PSD by (A) water tank and (B) instantaneous awakening methods on the percentage ratio of various phasese of SWC.
Columns on A: 1-baseline, 2, 3, 4 and 5- postdeprivation period during first, second, third and fourth days respectively.
Columns on B: 1-baseline, 2 and 3- during deprivation days, 4 and 5 in the postdeprivation period.
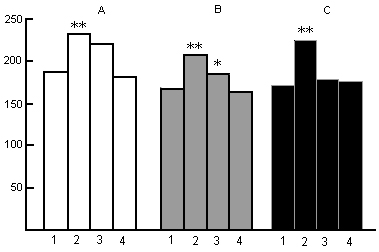
Figure 10. Influence of PSD by water tank method on the heart rate in the postdeprivation SWC at wakefulness (A), SWS (B) and PS (C).
On the ordinate: frequency of heart beats.
Columns: 1-baseline, 2-, 3- and 4- first, second and third days of postdeprivation period respectively.
(*P<0.02, **P<0.01, compared with baseline)
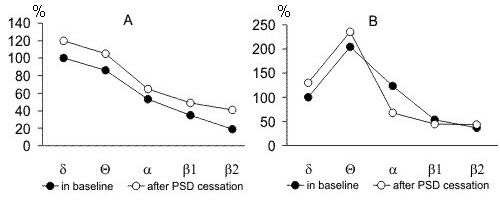
Figure 11. The pronounceness in the amplitudes of d, Q, a, b1 and b2 rhythms in the recording of baseline SWC and recordings of postdeprivation period after cessation of PSD procedure by water tank method. A, during SWS in the sensorimotor area of the neocortex; B, during PS in hippocampus. (the amplitude of baseline d rhythm is taken to be 100%).
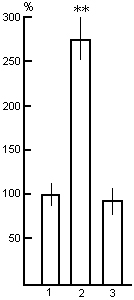
Figure 12. Influence of PSD by the instantenous awakening method on the frequency of PS onset. Columns: 1-in baseline (is taken to be 100%); 2-in the time course of deprivation procedure, 3-in the postdeprivation period after PS rebound ending, when PS amount returns to baseline level.
(**P<0.01, compared with baseline)
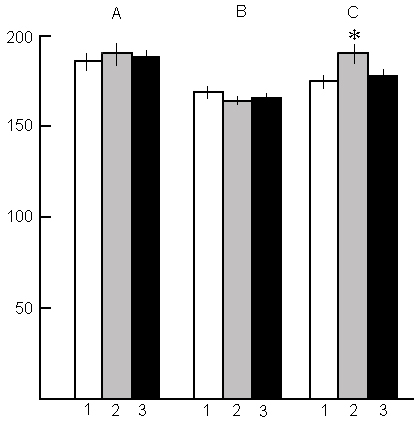
Fig. 13. Influence of PSD by the instantenous awakening method on the heart rate at wakefulness (A), SWS (B) and PS (C) during deprivation and postdeprivation period.
Columns on A and B: 1-baseline; 2-in the deprivation process, 3 - in the recovery period;
Columns on C: 1-baseline; 2-in the PS rebound; 3-after PS rebound ending, when PS amount returns to baseline level.
(*P<0.02, compared with baseline)
Correspondence: Oniani Tengiz, Prof.,
Department of Neurobiology of Sleep-Wakefulness Cycle,
I.S. Beritashvili Institute of Physiology, Georgian Academy of Sciences,
14, L. Gotua str., Tbilisi, 380060, Georgia.
E-mail: nswc@neurobiology.ge












