Neurobiology of Sleep-Wakefulness Cycle 1(1): 14 - 27, 2001
Printed in Georgia. All rights reserved.
© 2001 NSWC
IS SELECTIVE AND COMPLETE PARADOXICAL SLEEP DEPRIVATION POSSIBLE?
T. Oniani; L. Maisuradze; N. Lortkipanidze; M. Mgaloblishvili-Nemsadze; L. Oniani; M. Eliozihvili; N. Oniani
Department of Neurobiology of Sleep-Wakefulness Cycle, I.S. Beritashvili Institute of Physiology, Georgian Academy of Sciences, Tbilisi
Accepted in revised form 12 October 2001; recieved 2 August 2001.
Summary
A new method of selective and complete paradoxical sleep deprivation (PSD) is proposed, involving the replacement of paradoxical sleep (PS) with episodes of wakefulness equivalent in duration. In contrast to the "classical" method of momentary awakening of the animal, the new method does not lead to (1) the accumulation of a need for PS, associated with more frequent onsets of PS during deprivation; (2) PS rebound in the postdeprivation period; (3) dissociation of PS components, i.e. their occasional occurrence in the other phases of the sleep-wakefulness cycle (SWC) due to the phenomenon of self-deprivation; (4) increase in the rate of ponto-geniculo-occipital (PGO) spikes, rapid eye movements (REMs) and heart rate during PS phases in the postdeprivation rebound period. The application of this selective and complete PSD technique does not cause noticeable changes in the learning processes, functional state and integrative activity of the brain; this guaranteeing its successful use for the treatment of some psychoneurological disturbances in clinics.
Key Words: Paradoxical sleep, PS Deprivation, Accumulation, Need for PS, Wakefulness.
Introduction
In order to elucidate the functional significance of sleep a non-pharmacological method of its deprivation involving maintenance of prolonged waking state was used early as in 1894 by Manacein (see Kleitman 1963). For yet unknown causes the initial results seemed to be dramatic - under the activation of many day sleep deprivation the experimental dogs often died. Further investigations did not confirm a harmful effect of total sleep deprivation on animals, and the method of deprivation does remain the most effective in the study of sleep functions.
Research in this direction has been carried on with extreme intensity ever since it was established that sleeping state in terms of its neurophysiological parameters consists of two phases (Tskipuridze 1950; Aserinsky, Kleitman 1953; Dement and Kleitman 1957; Dement 1958; Jouvet and Michel 1960): ortodoxal or slow-wave sleep (SWS) and paradoxical sleep (PS) or rapid eye movement one. The paradox of the latter lies in the fact that during it, against the background of deep behavioral sleep, brain structures (particularly the forebrain) are activated just like in the waking state (Dement 1958; Jouvet et al. 1959; Oniani et al. 1970, 1978), thus creating an optimal background for the development of dreamings with their characteristic mental processes (Aserinsky and Kleitman 1953; Dement and Wolpert 1958). As a rule upon awakening from PS the subjects tell about the occurrence of dreams. The fact that 20% of the subjects tell about dreams being awakened from SWS can be accounted for the retention by memory of dreams occurring during SWS as well. It is just due to the mentioned peculiarities that PS phase has attracted special attention of researchers and numerous experiments have been devoted to the elucidation of its functional significance. Of major importance here was also the circumstance that non-pharmacological deprivation methods turned out to be most effective just with respect to PS phase. Momentary awakening of the sleeper leads to the cessation of this state and its transition to more or less prolonged episode of wakefulness followed by the restoration of SWS-PS cycle.
Adequate as it is, the method of selective deprivation by momentary forced awakening has to be however, very laborious, and thorough therefore the researchers began searching for other methods which would provide prolonged PS deprivation (PSD) without constant experimentator's control. Initially, a water tank procedure was proposed as such a method, first applied to cats in the laboratory of M. Jouvet (Jouvet et al. 1964) and to rats in the laboratory of W. Dement (Morden et al. 1967). At first this method found wide application, but later on it was shown that the water tank causes a permanent stress in the experimental animals, which can result in the occurrence of non-specific processes in the brain, leading to the disturbances of its normal integrative activity (Dement et al. 1967; Vogel 1975; Ogilvie and Broughton 1976; Fishbein and Gutwein 1977; Kovalzon and Tsibulski 1984; Oniani 1984, 1985; Oniani and Lortkipanidze 1985). Under these conditions it is difficult to distinguish between effects due to PSD and those caused by stressful situation, and consequently on the basis of data obtained it is hardly possible to draw a certain conclusion about the PS functions.
In order to assess the effects of stress due to water tank procedure on the animals special control experiments have been carried out (Mendelson et al. 1973) in which experimental animals were placed on small pedestals in the water tank and the controls - on large ones. Thus, the experimental animals woke up at the onset of each PS for fear of falling into the water due to atonia of skeletal muscles, accompanying PS, i.e. they were PS deprived, whereas the control animals did not undergo total PSD, though they allegedly experienced the same stress situation as experimental animals. The comparison of the data obtained in both groups of animals have been expected to make it possible to determine the part of PSD per se. Unfortunately these experiments, however, could not clarify the confusion. The point is that water tanks having the same parameters, except for dimension of the pedestals, can cause stressful conditions of different intensity. The reason lies at least in three circumstances.
1. Apart from constant tension resulted from general stress situation in the water tank, the experimental animals, placed on small pedestals, experience an increased fear of falling into the water at the frequent onset of each PS, whereas the same does not occur in the control animals as they are allowed to sleep and have PS partly.
2. In the control animals, as compared to the experimental ones, the behavioral and EEG sleep components develop rather more synchronously without significant dissociation between these components.
3. The control animals, thanks to a normal structure of the sleep-wakefulness cycle (SWC) (they have both sleep phases), sleep much more and wake less than experimental animals, and the sleeping state is known (Oniani and Lortkipanidze 1985) to sharply decrease general emotional tension of the organism induced by a stressful situation in the water tank. Thus, the visible difference of changes in the brain integrative activity between control and experimental animals should not be ascribed only to the presence of PS in the former and to its absence in the later.
These is a doubt about the adequacy of the above described control in application of water tank PSD procedure that appears to stimulate the search for other automatized methods ensuring more proper control experiments. However, neither the disk technique offered by Rechtshaffen et al. (1983); nor the pendulum method developed by Van Hulzen and Coenen (1980) can warrant an appropriate control procedure allowing to discriminate effects caused by stressful situation (Feinberg 1999) intrinsic in each of the above methods, and those produced by selective PSD per se. Besides, these methods suffer of certain shortcomings, that hamper the analysis of the data obtained.
Therefore it is our firm conviction that an old "classical" method, involving PS interruption by behavioral awakening of the sleeper immediately at PS onset under the control of the experimentator, though being laborous, remains the most adequate for PSD. It found wide application in many laboratories all over the world (Beersma et al. 1990; Camarini and Benedito 1997; Endo et al. 1998; Roth et al. 1999; Vu et al. 1999), including ours (Oniani 1985; Oniani et al. 1988a; 1988b; 1988c), and it has provided significant and valid findings, pointing to an important role of PS in the regulation of normal activity of the brain and the SWC as a whole.
Two findings should be singled out: 1. PSD by momentary behavior awakening immediately at the PS onset, that is there occurs sharp acceleration of PS onsets and, accordingly, an increase in the application of the arousing stimulus. 2. In the postdeprivation SWC there is a considerable increase in number of PS phases per time unit, their mean duration, as well as in the intensity of each phase, expressed by the aggravation of all the neurophysiological parameters, characteristic of the brain state in the given phase. All this leading to the changes in the interrelations between SWC phases in favour of PS, and thereby to the disturbance of a normal structure of the SWC.
These two findings indicate the accumulation of specific need for PS resulted from impossibility to satisfy it during deprivation, whereas in the postdeprivation period there occurs the satisfaction of PS need through its rebound.
In its turn, accumulation of specific PS need resulted in a considerable disturbance of the normal SWC structure both in the course of deprivation and in the postdeprivation period. During deprivation, apart from above mentioned strongly quickened onset of PS, they are expressed in the following:
1. Whereas normally PS occurs following a certain amount of deep SWS (DSWS), in the course of deprivation it can develop both against the background of light SWS (LSWS) and, which is special interest, during the wakefulness episode occurrence following PSD.
2. During PSD the synchronous occurrence of various neurophysiological parameters of PS is disturbed. Thus ponto-geniculo-occipital (PGO) spikes, characteristic of PS, can appear during DSWS (Dement 1972) and hippocampal theta rhythm can occur against the background of synchronized electrical activity of the neocortex, typical of DSWS.
3. During these occurs the phenomenon of the self-deprivation, formed in the same way as classical defense conditioned reaction (Oniani et al. 1988c), it being in the postdeprivation period retained for a certain time in the PS rebound phase. While in the postdeprivation period the disturbance of normal structure of the SWC is revealed by: drastic change of normal ratio of different phases at the expense of the PS volume increase; a considerable alternation of all the neurophysiological and behavioral parameters of PS (strengthened hippocampal theta rhythm; more frequent PGO spikes; accelerated fluctuation of heart rate, quickened rapid eye movements (REMs) and sporadical wincing of the solitary skeletal muscles; vorse atonia etc.(Oniani et al. 1988a; 1988b; 1988c)). All these signs do not testify to a non-stress origin of the new technique, however the stress evoked by it is considerably weaker than that of water tank (Jouvet 1965), disk (Rechtshaffen et al. 1983) and pendulum (Van Hulzen and Coenen 1980) methods.
As PSD is being already applied for clinical treatment of some psychoneurological disorders, causally related to the occurrence of this phase (endogenous depression, narcolepsy and others (Schultz 1982; Vogel et al. 1988; Vu et al. 1999)), accumulation of specific PS need during its deprivation and increase of both intensity and duration of PS phases in the postdeprivation period undoubtedly restrict the practical worth of this method. It is obvious, that whereas during the deprivation this procedure can have a positive effect, within the postdeprivation period due to above mentioned changes in PS parameters the effect can prove to be opposite.
This situation confronts the sleep investigators with the problem to find such non-pharmacological PSD method which application would not lead to substantial accumulation of PS need during deprivation and subsequently the disturbance of a normal structure of the SWC.
Accordingly, the question raises whether deprivation can be accomplished without above consequences in selective and complete PSD.
We are optimistic in answering these questions positively, which is also supported by several early (Oniani et al. 1985; Oniani 1986; Oniani et al. 1988a; 1988b; 1988c; Maisuradze et al. 1999) mentioned facts:
1. The sequence of phases in the normal SWC has strong regularities - PS occurs against the background of SWS and not wakefulness; moreover, a certain duration of SWS is required in order to trigger PS (Svorad and Karmanova 1966; Jouvet 1967, 1974; Ursin 1968; Oniani 1980). However, in the course of normal SWC for some reasons a well developed phase of SWS occasionally transfers to wakefulness phase rather than to PS and if the length of this wakefulness episode is approximately equivalent to mean duration of PS in this animal, the latency to the subsequent PS onset is twice as long. Thus, the impression is created that the episode of wakefulness of a certain duration can replace PS phase.
2. The same regularity can be frequently observed during PSD by the method of momentary awakening. When animals are aroused from the emerging PS by means of awakening stimulus, at first naturally, a more or less prolonged episode of behavioral wakefulness occurs, after which SWS is restored and only then the subsequent PS is triggered. These wakefulness episodes are usually short (5-10 sec), but occasionally they can last some minutes. If the length of this wakefulness episode equals approximately to a mean duration of PS phase in this animal, the occurrence of the next PS is postponed for the same time as if a full PS phase would have occurred. Thus, in this case the impression is again created that equivalent-in-duration wakefulness episode can replace PS phase.
3. When for some reasons, in the postdeprivation period a prolonged wakefulness phase at first develops, it considerably reduces PS rebound.
4. Our keen attention to the above facts has been apparently stimulated by the knowledge of the competitive relationship between wakefulness and PS which is more or less evident under different conditions in the course of the SWC in the animals (Dement 1972; Oniani et al. 1988b). Under the influence of the above mentioned facts arise question as to whether the wakefulness episodes equivalent-in-duration to PS phases can replace them in the SWC so that neither accumulation of specific PS need during deprivation, nor the PS rebound in the postdeprivation period occur.
To answer this question a series of experiments has been designed. That will be described bellow.
Methods
The experiments were conducted on 20 puberate cats (weighing 3-4 kg) divided into four groups (five animals in each). Every single group was subjected to PSD by means of "classical" method of momentary awakening at each PS onset (Dement 1960) and a new method of replacing PS phases by wakefulness fragments of equal PS mean duration in the baseline SWC.
The groups of animals were distinguished in accordance with the duration of the deprivation procedures: in the first group it lasted for 12 hours, in the second - 24 hours, in the third - 48 hours and in the fourth one procedure took 72 hours. To each group of cats PSD of various duration through different methods were applied three times.
Steel electrodes were chronically implanted under Nembutal anesthesia (30-35 mg/kg) in various brain structures (neo- and archipaleocortex) and in cervical and oculomotor muscles for the polygraphic recording of the SWC, as well as in various nuclei of the hypothalamus and mesencephalic reticular formation (MRF) for the stimulation; PGO spikes were recorded from lateral geniculate nucleus (LGN) of brain (Coordinates are taken from the atlas of Jasper and Ajmone-Marsan (1954)). After a 5-8 day postoperative recovery period, experiments involving the monitoring (on a 16-channel EEG of the firm "Medicor") of the SWC, and selective PSD were carried out in a special experimental chamber, where the acquisition of instrumental alimentary reflexes could be performed. Monopolar electroneocorticographic and electrohippocampographic recordings were obtained with an indifferent electrode placed either in the occipital bone of the skull or in the frontal sinus. An electromyogram and eye movement recordings were obtained via bipolar electrodes.
The schematic representation of the series of experiments with the application of selective PSD is shown in Fig. 1. The changes in the SWC structure under the action of I-selective PSD by momentary awakening (Fig. 1B) and II-selective PSD by awakening and further maintenance of wakefulness for a period equal to the mean duration of PS (Fig. 1C) were studied in the same chamber-adapted cats. The duration of fragments of evoked wakefulness was equal to the mean duration value of background PS phases (of about 8-10 minutes). Depending on the emotional tension of the induced wakefulness, the character of the mentioned state was determined by a behavioral reaction (attention and vigilance, in particular), as well as by the hippocampal theta rhythm that appeared in response to a sensory stimuli or direct electrical stimulation of activating brain structures. Different levels of wakefulness could be characterized as "non-emotional" wakefulness, "active" wakefulness with a certain moderate emotionality and "emotional" wakefulness with expressed emotionality. Changes produced by these two PSD procedures in the SWC both during deprivation and postdeprivation period were compared with the baseline SWC (Fig. 1A). After the cessation of PSD of one or another duration via one or another method, the SWC was continuously recorded during 24 hours, and the registration of the cycle during further several days (3-4) was performed only in the daytime. It was possible, in that way, to determine the PSD aftereffects, as well as to compare the effect of different PSD procedure.
It was borne in the mined that the first series of experiments would demonstrate an accumulation of need for PS during the period of its deprivation, which must be manifested in a acceleration of PS onsets and postdeprivation increase in its total amount. The second series of experiments was conducted to answer the question whether wakefulness episodes equal to mean PS duration are able to fully replace PS phases in the SWC. In addition, it was necessary to elucidate the character of the influence of selective PSD, employed in the above series of experiments on the functional state and integrative activity of the brain.
In order to define, via different methods, the effect of PSD on emotional-motivated behavior, the thresholds of electrical stimulation of ventromedial and lateral hypothalamus have been determined to induce orientation reaction as well as the reaction of anxiety, aggression, fear and alimentary behavior. CNS excitation under the influence of PSD was jugged on through one or another method in respect of the thresholds determination at the electrical stimulation of dorsal hippocampus, in dispensable induce therein local epileptiform discharge in the process or after the termination of a special type deprivation, comparing them to those inducing the mentioned discharges before the deprivation procedures started.
Electrical stimulation of the brain structures (dorsal hippocampus, different areas of the hypothalamus and MRF) has been performed by bipolar electrodes with the help of rectangular electric impulses, applaid from a high frequency generator output. The parameters (stimulus intensity, duration and frequency) appeared to be variable over a wide range (0,1 - 10 V; 0,5 - 5 msec; 0,2 - 500 Hz). High-frequency stimulation was used in order to obtain both isolated EEG activation and behavioral wakefulness.
With the view to study the effect of PSD on learning, a special series of experiments has been run using a special experimental chamber with two sections; hind - starting compartment, where the animal was placed during the intertrial period, and a front one with the area 1m2 having feeders over the front edges of the side-walls lifting the suspended doors with an instrumental pair movement the animal was able to get the reinforced portion of meat from the feeder. Tone of 500 Hz and clicks served as a conditioned signal, and their generators were fixed to the ceiling of the front wall. Against the background of the presentation of one or another signal, the door opened and the cat was taken to a signaled feeder. During a one day session a 10-fold combination of each sound signal with food reinforcement was produced. Learning was considered to have been terminated after there was a 100% level of conditioned signal discrimination, i.e. when the animal faultlessly made for the exact (correct) feeder in response to a conditioned signal. The experiments aimed at the acquisition of food-obtain reflexes started at one and the same time of the day, from 10 to 12 a.m.
The data were statistically treated. Mean values were calculated, their standard deviations and the validity of difference in the mentioned mean values were checked by Student's t-test.
During PS, the quantity of its phasic components was measured, in particular, PGO spikes and REMs (analysis epoch - 20 sec) as well as the rate of cardiac (heart) contractions (analysis epoch - 5 sec). These parameters were selectively calculated in number.
PSD was affected by awakening the animal following the transition of SWS into PS. The onset of PS was determined by the changes in the electric activity of the sensorimotor area of the neocortex, hippocampus, LGN and neck muscle as well as in eye movements.
The animals were aroused from PS by suprathreshold sensory stimuli (electric stimulation of the limb skin, pulling the rope tied to the animal's limb, knocking at the chamber walls, a relatively loud tone, etc.) or by direct electric stimulation of the mesencephalon and diencephalon.
At the termination of the experiments, the cats were sacrificed under Nembutal anesthesia, the brain was hardened by perfusion through the carotid artery with Neutral Formalin, removed and again placed in Formalin. The localization of the deep electrodes was verified on serial, 20-50 micron sections of the brain.
Results
1. Changes in the polygraphic pattern of the SWC during deprivation.
In the conventional experimental chamber, to which the animal is well adapted, the behavioral and EEG components of each phase develop synchronously. During DSWS, a usual precursor of PS, there occur slower and high-amplitude electric potentials both in the neocortex and in the hippocampus against the background of neck muscle atonia (as the animal is lying curled up, with its head on a firm support) and absence of REMs (Fig. 2A). Transition from SWS to PS is, as a rule, characterized by more or less brief fragment, when the high-amplitude slow potentials are gradually suppressed in the neocortex as well as in the hippocampus (Fig. 2B-at the end of the recording). Following this fragment, in the hippocampus, there elaborates theta rhythm reaches its maximum value at the development of REMs (Fig. 2C - at the beginning of the recording). The very first evidence for the forthcoming PS onset are PGO spikes. They are developed as single discharges even against the background of DSWS, with delta rhythm most of all expressed in the cortical structures (Fig. 2A). Later on PGO spikes, again on the background of DSWS, become more frequent (Fig. 2B) and with PS onset start developing as group discharges (Fig. 2C). All these parameters indicate the valuable elaboration of PS development. If at this moment an applied sensory or direct electrical stimulation causes momentary awakening of the animal, a sharp suppression of the hippocampal theta rhythm, partial restoration of the skeletal tonus and disappearance of REMs and PGO spikes will constitute the most important polygraphic events (Fig. 2C). The heart rate does not undergo any considerable changes. After momentary awakening from the PS onset, the wakefulness fragment becomes typically short (within 10 sec) with following SWS restoration.
With the aim of further retainment of wakefulness to replace PS repeated sensory stimuli or electric stimulation of activating brain structures have to be applied. Depending on the character of the enforced wakefulness, the electric activity of the hippocampus will be correspondingly affected. When "quiet" wakefulness is maintained, the theta rhythm is irregular, and in the case of "active" wakefulness a regular theta rhythm is observed. When "active" wakefulness with the manifestation of motivational behaviors (attention, alarm) is induced in response to the electric stimulation of the activating structures of the brain stem, the intensity of the hippocampal theta rhythm may reach the level characteristic of PS. Thus the two different types of PSD seem possible to be compared: by means of cessation of PS initial phases or/and momentary awakening in response to a single sensory or electrical stimulation of the activating structures of the brain (i.e. "classical" method), when the wakefulness fragment is characteristically short with subsequent rapid restoration of SWS; or through further preservation retainment of wakefulness continuation within average duration of PS phase of the background SWC, i.e. when PS phases are replaced by wakefulness of equal to average value PS duration in the background cycles of the same animal.
A typical event during PSD by means of momentary awakenings is a gradual reduction of the latency of PS onsets. If, at the beginning, the restoration of SWS is required for the subsequent PS onset, then, in the course of a long-term deprivation, PS is capable of appearing during the just restored LSWS (Fig. 3A), and even during "electroencephalographic" ("EEG") wakefulness (Fig. 3B). This leads to a sharp acceleration of PS onsets and, accordingly, to increased pairings of PS onset with the arousing stimulus.
The next characteristic consequence of more or less prolonged PSD by the "classical" technique is the occurrence of the phenomenon of self-deprivation (Oniani et al. 1988c). The essence of this phenomenon is that at the initial moments of brief PS episodes the animal awakens without any sensory stimulation or electric brain stimulation. Moreover, this self-deprivation may occur both when PS is triggered following SWS, and when it occurs during LSWS (Fig. 3C) or during "EEG" wakefulness (Fig. 3D).
A different picture is observed when the animal is aroused at the exact onset of PS, and wakefulness is enforced for the mean duration of PS, that is, PS phases are replaced with wakefulness. In this instance, acceleration of PS onsets - against the baseline cycle - is less pronounced than in the case of PSD by momentary awakenings, or is totally absent. The degree of this effect depends on the level of wakefulness produced. The enforcement of "non-emotional" wakefulness may result in a slightly increased frequency of PS onsets (Fig. 4C) as compared to the baseline (Fig. 4A), but significantly lower than during PSD by momentary awakening (Fig. 4B), whereas in the case of "active" and "emotional" wakefulness being induced, the number of PS episodes in the sleep-wakefulness cycle is well below (Fig. 4D, E) that seen in the baseline cycle (Fig. 4A).
2. Changes in the structure of the postdeprivation SWC.
More or less prolonged (12, 24, 48 and 72 hours) PSD by momentary awakening produces clear-cut quantitative changes in the PS frequency and duration, resulting in an increase in PS amount without marked changes of SWS. A selective PS rebound is particularly well seen within the first 6-8 hours following the cessation of the 24 hours deprivation procedure (Fig. 5B). Even after the cessation of a 72 hour deprivation procedure under the "classical" method, the PS rebound lasts merely within 6-8 hours, with the restoration of its background value after that. The recording made in subsequent several days demonstrated that PS rebound was not delayed, since it did not occur in the SWC, registered 24 hours after the termination of PSD. Against the background of PS rebound in the postdeprivation period the emerging PS phases are frequently interrupted due to a kind of self-deprivation. These self-deprivations at first terminate in behavioral arousal, but afterwards PS episodes may be interrupted without behavioral awakening. In the postdeprivation period brief PS episodes may occur and terminate during SWS without producing considerable electroneocorticogram desynchronization; thus, the impression is created that some PS components (hippocampal theta rhythm and eye movements in our case) may intrude upon SWS.
However, if PSD is conducted in such a way as to replace PS phases with wakefulness episodes, postdeprivation PS rebound is lesser or absent altogether and occasionally even a decrease in PS amount (Fig. 5E) is observed. This depending on the character of the wakefulness induced. A quiet "non-emotional" wakefulness being elicited, postdeprivation PS rebound decreases (Fig. 5C), compared with "classical" method (Fig. 5B). If PS phases are replaced with episodes of "active" wakefulness (arousing stimulus-induced state of attention and vigilance), no increase in PS amount (Fig. 5D) in the postdeprivation period is observed (compared with baseline (Fig. 5A)).
An analysis of the polygraphic patterns reveals other changes in the postdeprivation SWC. Thus, in the case of PSD by momentary awakening during a postdeprivation selective PS rebound there is a considerable increase in the heart rate (Fig. 6A, grey column), REMs (Fig. 6C, grey column) and PGO spikes (Fig. 6B, grey column) as compared to the baseline patterns (Fig. 6A, B, C light columns). With the cessation of PS rebound the above components revert to the baseline values. The above postdeprivation alteration of the heart rate (Fig. 6A, black column), REMs (Fig. 6C, black column) and PGO spikes (Fig. 6B, black column) are absent when PS phases are replaced with "active" wakefulness fragments.
3. Changes in the excitability of various brain structures under the action of PSD.
The excitability of extensive cortical and subcortical (hippocampus, hypothalamus, MRF and some others) brain structures was studied by determining the electrical stimulation thresholds evoking activation of the electroneocorticogram and electrohippocamprogram during all the above deprivation manipulations. Account was also taken of the fact that in response to cortical and some subcortical stimulation diffuse activation of the electroneocorticogram can be produced by the excitation of nonspecific activating mesodiencephalic structures.
A careful comparison of the electric stimulation thresholds throughout 3-day PSD by "non-emotional" awakening and 24 hour postdeprivation period with those prior to the deprivation procedure showed that the excitability of most brain structures is not markedly affected under the action of PSD. Only a slight nonsignificant decrease in the thresholds of electric stimulation of the MRF (Fig. 7A) during the first postdeprivation hours - preferentially in PS - was observed, the same being revealed in the hippocampal theta rhythm changes.
No marked changes were observed in the thresholds, for epileptiform discharges evoked by direct electrical stimulation of the hippocampus either during prolonged PSD or in the postdeprivation period (Fig. 7B).
3-day selective PSD both by momentary awakening and through replacing PS phases by wakefulness had no considerable effect on biological motivations. This was manifested, firstly, in the lack of signs of hyperphagia and, secondly, in the fact that electric stimulation thresholds of hypothalamic motivating structures for evoking feeding behaviour (stimulation of the lateral hypothalamus) (Fig. 7D) and aggressive reaction (stimulation of the ventromedial hypothalamus (Fig. 7C) remained unaffected both during deprivation and in the postdeprivation period.
4. PSD effects on learning.
Special significance is attached to the PSD effect on learning and memory. On the basis of the data obtained, the widespread theory was formulated according to which PS plays a specific role in the memory organization through regulation of recent trace consolidation and transfer of short- into long-term memory (Pearlman and Greenberg 1973; Fishbein and Gutwein 1977; Smith 1985, 1993, 1996).
To test this theory we undertook the study of the effect of selective PSD by the "classical" method and by the new method of PS replacing with equivalent-in-time episodes of wakefulness on the acquisition of instrumental alimentary reflexes to two feeders with sound discrimination. In a special experimental chamber the acquisition of instrumental alimentary reflexes was effected by multisession learning. In each session, running from 10 to 11 a.m., sound signals were 10 times combined with food reinforcement from corresponding feeders. Following the learning session till the next morning the cats were subjected to PSD by the above procedures until 100% discrimination of conditioned signals was reached. The acquision of alimentary instrumental reflexes in cats deprived of PS by both procedures was found to proceed as successfully as in the controls (Fig. 8)
Discussion
The analysis of data obtained shows that although the "classical" PSD procedure makes it possible to delay the development of full-fledged PS phases for fairly long duration, during it there occurs an accumulation of an unsatisfied need for PS. This in turn leads to a sharp acceleration of PS onsets through-out deprivation and to selective rebound in the postdeprivation period. Moreover, due to accumulation of the need for PS there may also occur dissociative processes described in the literature (Dement 1972) and allegedly indicative of the possible occurrence of some PS components in other phases of the SWC. In analyzing the dissociative aspects of selective PSD, one should apparently take into account the participation of the self-deprivation phenomenon (Oniani et al. 1988c) in these processes. The point is that self-deprivation of PS may occur without any clear-cut behavioral reactions. Occasionally even a genuine restoration of neck muscle tonus is absent. In such cases PS episodes can begin and cease both during slow electrical activity of the neocortex and during desynchronization of the electroneocorticogram. Hence a misleading impression is created of an isolated occurrence of PS components in other phases of the SWC.
Thus, arousing the animal at each onset of PS phase in the SWC by the so-called "classical" method does not ensure selective and complete PSD.
In contrast to the "classical" method, neither acceleration of the onsets of PS during its deprivation non selective PS rebound in the postdeprivation period are observed when PSD by replacing PS within the SWC with equivalent-in-time episodes of "active" wakefulness is used. There is no increase in the frequencies of PGO spikes, REMs and heart rate either. Moreover, no phenomenon of PS self-deprivation or dissociation of particular components of this physiological brain state, resulting in significant disturbances of a normal SWC, can be seen. All these findings indicate that selective and complete PSD can be accomplished only through PS replacement with equivalent-in-duration episodes of "active" wakefulness.
Of great importance for the elucidation of the functional significance of PS as well as for the assessment of PSD methods with regard to clinical practice is the circumstance that selective and complete PSD by replacement of PS with wakefulness does not induce considerable changes in the functional state and integrative activity of the brain. The absence of the negative effect of PSD by momentary awakening on the integrative activity of the brain may be explained by the fact that just then the mechanisms of PS continue working, at least, partly on the background of other phases (which is evidenced by the presence of dissociation process) and, thus, may accomplish their function in the sense of memory traces consolidation. However, such a conclusion seems to be hardly even possible in light of the results of the new method of PSD, since PS phasic components do not interfere the other phases of sleep, which points, in this case, to the absence of PS mechanisms functioning.
In view of the foregoing, it seems guaranteed to conclude that:
1. Episodes of wakefulness (especially "active") replace PS phases and provide satisfaction of a need for PS;
2. Biological needs for PS and wakefulness might be identical in terms of neurochemical changes occurring in the brain during SWS. The last conclusion appears to be a most provocative speculation because, for the present, we may assume with certainly only the occurrence of a biological need for PS, and we have no data available for a biological need for wakefulness. But now reliable data on a biological need for wakefulness have been accumulated in our laboratory (Akhvlediani et al. 1988) which enable us to formulate the above assumption. The discussion of this problem brings about another question: on the background of which phase of SWS ought the biological need for PS to be formed. In the view of the above data the significance of wakefulness phase in this process is excluded, ascribing therefore much more importance to the meaning of SWS. If could be assumed that during the phase of wakefulness there takes place the formation of merely biological need for SWS since both for PS and wakefulness the biological need is formed precisely at SWS (Oniani et al. 2000). Further experiments should be aimed at bringing about more clearness in this question.
References
Akhvlediani, G., Oniani, T., Chikvaidze, V. The effect of some monoamine oxidase inhibitors on the sleep-wakefulness cycle of the cat. In: T. Oniani (Ed) Neurobiology of Sleep-Wakefulness Cycle. Metsniereba, Tbilisi, 1988: 423-431.
Aserinsky, E., Kleitman, N. Regularly occurring period of eye motility and concomitant phenomena during sleep. Science, 1953, 118: 273-274.
Beersma, D., Dijk, D., Block, C., Everhardus, I. REM sleep deprivation during 5 hours leads to an immediate REM sleep rebound and to suppression of non-REM sleep intensity. EEG Elin. Neurophysiol., 1990, 76: 114-122.
Camarini, R., Benedito, M. Rapid eye movement (REM) sleep deprivation reduces rat frontal cortex acetylcholinesterase activity. Braz. J. Med. Biol. Res., 1997, 30, 5: 641-647.
Dement, W. The occurrence of low voltage, fast, electroencephalogram patterns during behavioral sleep in the cat. Electroenceph. Clin. Neurophysiol. , 1958, 10, 2: 291-296.
Dement, W. The effect of dream deprivation. Science, 1960, 131: 1705-1707.
Dement, W. Sleep deprivation and the organization of the behavioral states. In: C. Clemente, D. Purpura, F. Mayer (Eds) Sleep and maturing nervous system. N.Y., London, Acad. Press, 1972: 261-319.
Dement, W., Henry, P., Cohen, H., Fergusson, G. Studies on the effect of REM deprivation in humans and in animals. In: Sleep and altered states of consciousness, proceedings of the association for research in nervous and mental disease. Baltimore, Williams and Wilkins, N.Y. 1967: 456-468;
Dement, W., Kleitman, N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility and dreaming. Electroenceph. Clin. Neurophysiol. , 1957, 9: 673-690.
Dement, W., Wolpert, E. The relation of eye movement body motility and external stimuli to dream content. J. Exp. Psychol., 1958, 55, 2: 549-553.
Endo, T., Roth, C., Landolt, H., Werth, E., Aeschbach, D., Acherman, P., Borbely, A. Selective REM sleep deprivation in humans: effects on sleep and sleep EEG. Am. J. Physiol., 1998, 274: R1186-R1194.
Feinberg, I. Delta homeostasis, stress and sleep deprivation in the rat: A coment on Rechtschaffen et al. Sleep , 1999, 22, 8: 1021-1025.
Fishbein, W., Gutwein, B. Paradoxical sleep and memory storage processes. Behav. Biol., 1977, 19: 425-464.
Jasper, H., Ajmone-Marsan, C. A stereotaxic atlas of the diencephalons of the cat. The National Research Council of Canada, 1954.
Jouvet, D., Vimont, P., Delorme, F., Jouvet, M. Etude de la privation selective de la phase paradoxale de sommeil le chat. C. R. Soc. Biol., Paris, 1964, 158, 4: 756-759.
Jouvet, M. Behavioral and EEG effect of paradoxical sleep deprivation in the cat. Proc. 23rd Int. Congr. Physiolog. Sci., Tokyo, 1965, 4, 87: 344-355.
Jouvet, M. The neurophysiology of the states of sleep. Physiol. Rev. , 1967, 47, 2: 117-177.
Jouvet, M. The role of monoaminergic neurons in the regulation and function of sleep. In: O.Petre-Quadens and J. Schlag (Eds) Basic Sleep Mechanisms. Acad. Press, New York, London, 1974: 207-237.
Jouvet, M., Michel, F. Délenchements de la "phase paradoxale" du sommeil par stimulation du trone cerebral chez le chat intact et mesencephalique chronique. C. R. Soc. Biol., Paris, 1960, 154: 634.
Jouvet, M., Michel, F., Courjon, I., Hermann, H. L'activite electrique du rhinencepale au cours du sommeil chez le chat. C.R. Soc. Biol., Paris, 1959, 153, 1: 101-105.
Kleitman, N. Deprivation of sleep. In: Sleep and Wakefulness. University of Chicago Press, Chicago, 1963: 215-323.
Kovalzon, V., Tsibulsky, V. REM sleep deprivation, stress and emotional behavior in rats. Behav. Brain Res., 1984, 14, 3: 235-245.
Maisuradze, L., Oniani, T., Lortkipanidze, N., Oniani, L., Eliozishvili, M. Is selective paradoxical sleep deprivation possible? Sleep Res. Online, Dresden, Germany, 1999, 2, (suppl. 1): 544.
Mendelson, W., Guthrie, R., Frederick, G., Wyatt, R. Should flower pots be used for flowers, pot or rats? In: M. Chase, W. Webb, R. Wilder-Jones (Eds) Sleep Research. Los Angeles, Brain Information Service (Brain Research Institute, University of California), 1973, 2:169.
Morden, B., Mitchell, G., Dement, W. Selective REM sleep deprivation and compensation phenomena in the rat. Brain Res., 1967, 5, 3: 339-349.
Ogilvie, R., Broughton, R. Sleep deprivation and measures of emotionality in rats. Psychophysiol., 1976, 13, 3: 249-260.
Oniani, T. The Integrative Function of the Limbic System. Metsniereba, Tbilisi, 1980, (in Russian).
Oniani, T. Does paradoxical sleep deprivation disturb memory trace consolidation? Physiol. Behav., 1984, 33, 5: 687-692.
Oniani, T. Paradoxical sleep and the regulation of motivational processes. In: T. Oniani (Ed) Neurophysiology of Motivation, Memory and Sleep-Wakefulness Cycle. Metsniereba, Tbilisi, 1985, 4: 9-58, (in Russian).
Oniani, T. Neurophysiological unalysis paradoxical sleep deprivation. Materials of Intern. Symposium "Neurobiology of Sleep-Wakefulness Cycle". Metsniereba, Tbilisi, 1986: 66-68.
Oniani, T., Lortkipanidze, N. Effect of paradoxical sleep deprivation on learning and memory. In: T. Oniani (Ed) Neurophysiology of Motivation, Memory and Sleep-Wakefulness Cycle. Metsniereba, Tbilisi, 1985, 4: 136-214.
Oniani, T., Lortkipanidze, N., Maisuradze, L. Neurophysiological analysis of deprivation of paradoxical sleep. "Topical problems of sleep physiology and pathology". I MMI, Moscow, 1985: 64-66, (in Russian).
Oniani, T., Lortkipanidze, N., Mgaloblishvili, M., Maisuradze, L., Oniani, L., Babilodze, M., Gvasalia, M. Neurophysiological analysis of paradoxical sleep deprivation. In: T. Oniani (Ed) Neurobiology of sleep wakefulness cycle. Metsniereba, Tbilisi, 1988a: 19-43.
Oniani, T., Lortkipanidze, N., Maisuradze, L., Oniani, L. Neurophysiological analysis of the effects of paradoxical sleep selective deprivation. J. Neurophysiologia , Kiev, 1988b, 20, 1: 20-28, (in Russian).
Oniani, T., Maisuradze, L., Lortkipanidze, N., Oniani, L. Paradoxical sleep self-deprivation in cats. J. VND, 1988c, 38, 2: 266-274, (in Russian).
Oniani, T., Maisuradze, L., Lortkipanidze, N., Mgaloblishvili, M., Chidjavadze, E., Oniani, L., Oniani, N., Babilodze, M. Total paradoxical sleep deprivation through partial deprivation of slow-wave sleep. Bulletin of the Georgian Acad. of Sci., 2000, 161, 1: 127-131.
Oniani, T., Molnar, P., Naneishvili, T. The nature of paradoxical phase of sleep. Fiziol. Zh. SSSR, imeni T.M. Sechenova, 1970, 56, 5: 689-695, (in Russian).
Oniani, T., Molnar, P., Naneishvili, T. The nature of paradoxical phase of sleep. Neuroscience Translation. Fed. Amer. Soc. Exp. Biol., 1978, 15, 1.
Pearlman, C., Greenberg, R. Posttrial REM sleep: A critical period for consolidation of shuttle-box avoidance. Anim. Learn. Behav., 1973, 1: 49-51.
Rechtschaffen, A., Gilliand, M., Bergmann, B., Winter, J. Physiological correlates of prolonged sleep deprivation in rats. Science, 1983, 221, 4606: 182-184.
Roth, C., Endo, T., Landolt, H., Wurth, E., Borbely, A., Achermann, P. Selective REM deprivation effect of frequent awakenings on sleep latency and intermittent sleep-time. Sleep Res. Online, Dresden, Germany, (suppl. 1), 1999, 2: 552
Schulz, H. REM-schlaf bei endogener depression. Biol. Psychiatr. Fortschr. Psychiatr. Forsch., Stuttgart, New York, 1982: 314-322.
Smith, C. Sleep states and learning: a review of the animal literature. Neurosci. Biobihav. Rev., 1985, 9: 157-168.
Smith, C. REM sleep and learning: some recent findings. In: A. Mofitt, M. Kramer and R. Hoffmann (Eds) The functions of dreaming. N.Y., SUNY Press, 1993: 341-361.
Smith, C. Sleep states, memory processes and synaptic plasticity. Behav. Brain Res., 1996, 78: 49-56.
Svorad, D., Karmanova, I. Synchronizing mechanisms in the release of the rhombencephalic phase of sleep. Nature, 1966, 212, 5063: 713-714.
Tskipuridze, L. Electrical activity of neocortex in normal cat during natural sleep. Works of the Inst. of Physiology, Acad. Sci. Georgian SSR, Tbilisi, 1950, 8: 209-225, (in Russian).
Ursin, R. The two stages of slow wave sleep in the cat and their relation to REM sleep. Brain Res., 1968, 11, 2: 347-356.
Van Hulzen, Z., Coenen, A. The pendulum technique for paradoxical sleep deprivation in rats. Physiol. Behav., 1980, 25, 5: 807-811.
Vogel, G. A review of REM sleep deprivation. Arch Gen. Psychiat., 1975, 32, 6: 749-761.
Vogel, G., Roth, T., Gillin, J., Mendelson, W., Buffenstein, A. REM sleep and depression. In: T. Oniani (Ed) Neurobiology of Sleep-Wakefulness Cycle. Metsniereba, Tbilisi, 1988: 187-215.
Vu, H., Roth, C., Mathis, J., Hurni, C., Achermann, P., Basseti, C. REM sleep regulation in narcolepsy: effects of selective REM deprivation. Sleep Res. Online, Dresden, Germany, 1999, 2 (Suppl. 1): 463.
Acknowledgments
This research was supported by the Japanese Government, under the ISTC grant G-391.
Figures
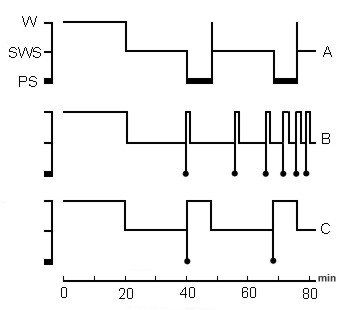
Figure 1. The schematic representation of PSD experiments by various methods.
Design of SWC structure during: A, baseline; B, PSD by "classical" method of momentary awakening; C, PSD by new method - through replacement of PS with fragments of wakefulness equivalent to baseline PS phases mean duration. Notation: W, wakefulness; SWS, slow wave sleep; PS, paradoxical sleep.
Under design - the time scale in minutes.
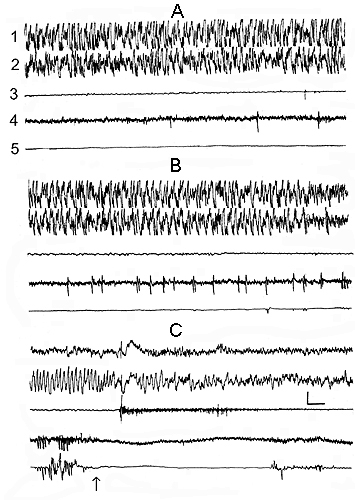
Figure 2. Change of the electrical activity of the neo- and archipaleocortex, skeletal muscle tone and eye movements at PSD.
A, DSWS; B, transition from DSWS to PS; C, PS and its deprivation through awakening in response to electrical stimulation of the lateral hypothalamus.
Leads: 1, sensorimotor area of neocortex; 2, dorsal hippocampus; 3, cervical muscle; 4, LGN; 5, oculomotor muscles. (the switch-on of electrical stimulation is indicated by the arrow). Calibration on this and Figure 3. is 200mV, 1 sec.
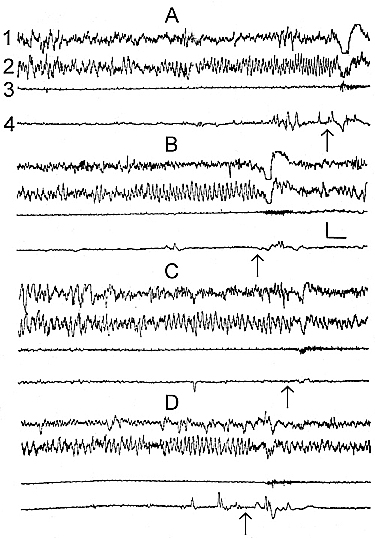
Figure 3. Changes of the electrical activity of the neo- and archipaleocortex, skeletal muscle tone and eye movements at PSD by "classical" method of momentary awakening.
Leads: 1, visual cortex; 2, dorsal hippocampus; 3, cervical muscle; 4, oculomotor muscles. A, transition of LSWS to PS and its deprivation; B, triggering and deprivation of PS against the background of "EEG" wakefulness; C, transition of LSWS to PS and its self-deprivation; D, triggering of PS against fragment of wakefulness and PS self-deprivation (the moments of PSD and PS self-deprivation are marked by arrows).
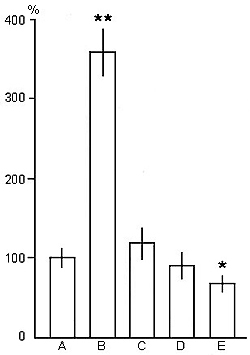
Figure 4. The effect of PSD by various techniques on the frequency of PS phases onset during deprivation procedures.
On the ordinate: amount of PS onset in percentage. A, in the baseline (is taken as 100 %); B, at PSD with momentary awakening method; at PSD through replacement of PS by: C, "non-emotional", D, "active" and E, "emotional" wakefulness fragments.
(The significance of difference between: A and B: **p<0,01; A and E: *p<0,05).
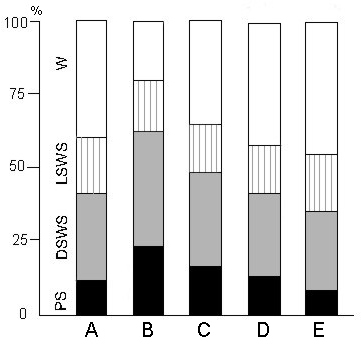
Figure 5. The effect of PSD by various methods on the percentage ratio of different phases in a 8 hr postdeprivation SWC.
A, baseline ratio; B, following PSD by "classical" method; following PSD through PS replacement with fragments of: C, "non-emotional"; D, "active" and E, "emotional" wakefulness.
(One of the typical results is presented).
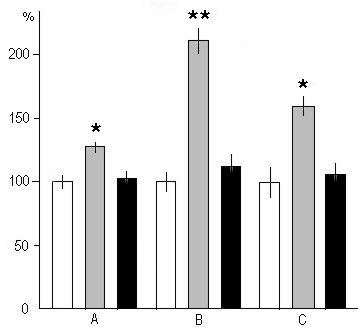
Figure 6. The effect of PSD by various methods on the postdeprivation PS quality.
A, heart rate; B, PGO spikes; C, REMs. On the ordinate: amount of these parameters at PS in percentage. Light columns - in baseline (is taken as 100 %); grey columns - following PSD by "classical" method; black columns - following PSD through replacement of PS by "active" wakefulness.
(* p<0.01, ** p<0.001 compared with baseline).
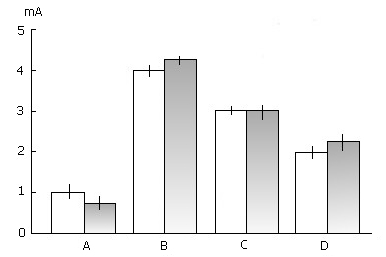
Figure 7. The effect of a 3-day PSD through PS replacement with fragments of wakefulness on the thresholds of electrical stimulation of the MRF (A), dorsal hippocampus (B), ventromedial (C) and lateral (D) nucleus of the hypothalamus for evoking the orientation reaction, epileptiform discharges, aggressive and alimentary reaction correspondingly.
On the ordinate: power of electric stimulation in mA. Light columns - before deprivation procedure; grey columns - after cessation of PSD.
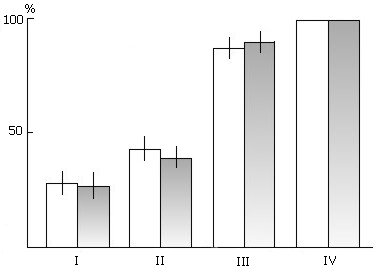
Figure 8. The effect of PSD by the replacement of PS with fragments of "active" wakefulness on the acquisition of instrumental alimentary reflexes to two feeders with sound discrimination.
On the ordinate: percentage of correct responses; on the abscissa: learning sessions (I, II, III, IV). Light columns in each pair: in the control group of animals; grey columns: in the experimental ones.
Correspondence: Oniani Tengiz, Prof.,
Department of Neurobiology of Sleep-Wakefulness Cycle,
I.S. Beritashvili Institute of Physiology, Georgian Academy of Sciences,
14, L. Gotua str., Tbilisi, 380060, Georgia.
E-mail: nswc@neurobiology.ge







